
ﮱ���Ϊ�����ܽ���������ҵ����Ũ�������-﮻Կ�(Li2Al2Si4O12��������þ����)��250��300�淴Ӧ������Li2SO4�������������Լ�MgSO4�ȣ��乤ҵ�����������£�
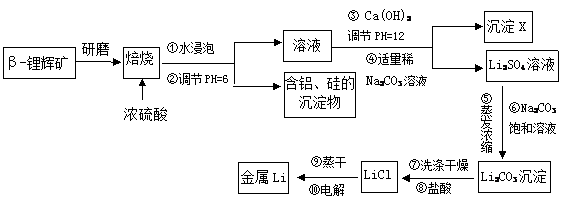
��֪�� Al3+��Mg2+������������ʽ��ȫ����ʱ����Һ��PH�ֱ�Ϊ5.2��12.4
(1)����������ʽ��ʾLi2Al2Si4O12����ɣ�_____________ ______��
(2)����X����Ҫ�ɷ���(д��ѧʽ)��______________��___ _____��
(3)д���ڢ۲�����������Ӧ�Ļ�ѧ��Ӧ����ʽ�� ��
(4)��������������ʹ�ò�ͬŨ�ȵ�̼������Һ��Ŀ�ģ�
��
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 235 |
| 92 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ﮱ���Ϊ�����ܽ���������ҵ�����������-﮻Կ�(LiAlSi2O6��������þ����)��250��300�淴Ӧ������Li2SO4�Լ����������MgSO4�ȣ��乤ҵ�����������£�

(1)����������ʽ��ʾLiAlSi2O6����ɣ�________��___________��
(2)����X����Ҫ�ɷ���(д��ѧʽ)________ ��________________��
(3)����������ʹ����Na2CO3��Һ����˵��ǰ��Ũ�Ȳ�ͬ��ԭ��:_____ ��___________��
(4)﮺������ڼ���ʱ�ܷ�Ӧ���ɰ�ɫ�����⻯ﮣ��⻯�����ˮ�������ܽⲢ�ͷų����������塣��д���⻯���ˮ��Ӧ�Ļ�ѧ����ʽ����______________��
(5)��������Li2C03��ȫ��Ӧ�����Һ���������ɵõ����壬�ٽ������ڵ������ﮡ����ʱ�����������л��������������ԭ����______��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�������ѧ�����꼶���Ĵο��� ���ͣ�ʵ����
ﮱ���Ϊ�����ܽ���������ҵ�����������-﮻Կ�(LiAlSi2O6��������þ����)��250��300�淴Ӧ������Li2SO4�Լ����������MgSO4�ȣ��乤ҵ�����������£�

(1)����������ʽ��ʾLiAlSi2O6����ɣ�___________________��
(2)����X����Ҫ�ɷ���(д��ѧʽ)________________________��
(3)����������ʹ����Na2CO3��Һ����˵��ǰ��Ũ�Ȳ�ͬ��ԭ��:
____________________________________________________________________��
(4)﮺������ڼ���ʱ�ܷ�Ӧ���ɰ�ɫ�����⻯ﮣ��⻯�����ˮ�������ܽⲢ�ͷų����������塣��д���⻯���ˮ��Ӧ�Ļ�ѧ����ʽ��_____________________________________��
(5)��������Li2C03��ȫ��Ӧ�����Һ���������ɵõ����壬�ٽ������ڵ������ﮡ����ʱ�����������л��������������ԭ����__________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com