
(13 ��)��һ�������ĩ����CaCO3��Na2SO4��KCl��Ba (NO3)2��CuSO4�е�����������ɣ�ȡ��Ʒ��������ʵ�飺
��1��д��ʵ������з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2�������ĩ��һ�������ڵ������� ��һ�����ڵ������� ��
��3���������ĩ���ܵ���������±��������Բ�������Ҳ�����ٲ��䡣��
| ��� | ��ѧʽ |
| �� | |
| �� | |
| �� | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13��)��һ�������ĩ����CaCO3��Na2SO4��KCl��Ba(NO3)2��CuSO4�е�����������ɣ�ȡ��Ʒ��������ʵ�飺

��1��д��ʵ������з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2�������ĩ��һ�������ڵ������� ��һ�����ڵ������� ��
��3���������ĩ���ܵ���������±��������Բ�������Ҳ�����ٲ��䡣��
| ��� | ��ѧʽ |
| �� |
|
| �� |
|
| �� |
|
��4�������һ��ʵ���һ��ȷ����������ɣ�����ʵ�鲽�衢����ͽ��ۡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ��������֪��ѧ�߶���ѧ�����п����Ŀƻ�ѧ�Ծ�(�������� ���ͣ��ƶ���
(4��)��һ�������ĩ����CaCO3��Na2SO4��KNO3��BaCl2��CuSO4�е�����������ɣ�ȡ��Ʒ��������ʵ�飺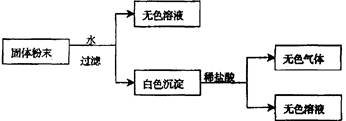
��ʵ������жϣ��ù����ĩ��
��1��һ�������� ��
��2��д����ɫ����ķ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������и�һ��ѧ��10���¿�����ѧ�� ���ͣ������
(13 ��)��һ�������ĩ����CaCO3��Na2SO4��KCl��Ba (NO3)2��CuSO4�е�����������ɣ�ȡ��Ʒ��������ʵ�飺
��1��д��ʵ������з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2�������ĩ��һ�������ڵ������� ��һ�����ڵ������� ��
��3���������ĩ���ܵ���������±��������Բ�������Ҳ�����ٲ��䡣��
|
��� |
��ѧʽ |
|
�� |
|
|
�� |
|
|
�� |
|
��4�������һ��ʵ���һ��ȷ����������ɣ�����ʵ�鲽�衢����ͽ��ۡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13 ��)��һ�������ĩ����CaCO3��Na2SO4��KCl��Ba (NO3)2��CuSO4�е�����������ɣ�ȡ��Ʒ��������ʵ�飺

��1��д��ʵ������з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2�������ĩ��һ�������ڵ������� ��һ�����ڵ������� ��
��3���������ĩ���ܵ���������±��������Բ�������Ҳ�����ٲ��䡣��
| ��� | ��ѧʽ |
| �� | |
| �� | |
| �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com