
��2L���ܱ������г���7.6mol NO��3.8mol O2���������·�Ӧ��
��2NO��g����O2��g�� 2NO2��g��
2NO2��g��
��2NO2��g�� N2O4��g��
N2O4��g��
���NO2��N2O4��Ũ�ȱ仯��ͼ��ʾ��0~10minά�������¶�ΪT1�棬 10min�����߲�ά���������¶�ΪT2�档����˵����ȷ���ǣ� ��
A��ǰ5min��Ӧ��ƽ������v��N2O4����0.18mol��L��1��s��1
B��T1��ʱ��Ӧ�ڵĻ�ѧƽ�ⳣ��K��0.6
C����Ӧ�١��ھ�Ϊ���ȷ�Ӧ
D������ʼʱ��������г���3.6mol NO2��2. 0mol N2O4��T1��ﵽƽ��ʱ��N2O4��ת����Ϊ10%

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A��Kspֻ�����ܵ���ʵ����ʺ��¶��й�
B������Ksp(ZnS)��Ksp(CuS)������ZnS������һ�������¿�ת��ΪCuS����
C�������������䣬����Ũ�ȸı�ʱ��Ksp����
D���������ܵ���ʣ�KspС�ģ��ܽ��һ��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������������������ɳ�����չ������ء�����˵����ȷ����
A.����CO2���ŷţ����Լ�������IJ���
B.����SO2���ŷţ����ԴӸ�������������
C.�²���̼��ά����һ�������л��߷��Ӳ���
D.CO2�ϳɾ�̼�����ɽ������ϣ���ʵ�֡�̼����ѭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��100mL 0.1 mol��L��1�������[NH4Al��SO4��2]��Һ����ε���0.1 mol��L��1 Ba��OH��2��Һ������Ba��OH��2��Һ���V�ı仯�����������ʵ���n�ı仯����ͼ��ʾ��������˵������ȷ���ǣ� ��
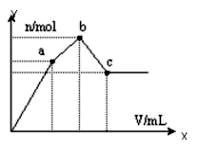 A��c����Һ�ʼ���
A��c����Һ�ʼ���
B��b�㷢����Ӧ�����ӷ���ʽ�ǣ�Al3++2SO42-+2Ba2++3OH�� =
Al��OH��3��+2BaSO4��
C��c�����Ba��OH��2��Һ�����Ϊ200 mL
D��a�����Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijǿ������ҺX�п��ܺ���Fe2+��A13+��NH4+��CO32�D��SO32�D��SO42�D��C1�D�е������֣���ȡX��Һ��������ʵ�飬ʵ����̼��������£�
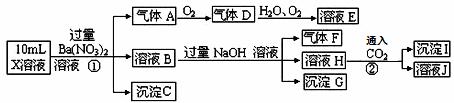
����˵����ȷ���ǣ� ��
A��X�п϶�����Fe2+��NH4+��SO42�D B����ҺE������F���ܷ�����ѧ��Ӧ
C��X�п϶�������CO32�D��SO32�D��C1�D D������I��A1��OH��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A��������ԭ��Ӧ�ı����Ƿ�Ӧǰ����Ԫ�ػ��ϼ۵�����
B��Ag����Cl��===AgCl���ķ�Ӧ���ڻ��Ϸ�Ӧ
C����1 L 1 mol��L��1��H2SO4��Һ��ȡ��10 mL����Һ������H��Ũ��Ϊ2 mol��L��1
D���������������ά��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʺ���ij��ԭ�Ӿ������ (����)��
A���۵�1 070 �棬������ˮ��ˮ��Һ����
B���۵�10.32 �棬Һ̬�����磬ˮ��Һ����
C��������CS2���۵�112 �棬�е�444.6 ��
D���۵�3 550 �棬��Ӳ��������ˮ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�������ݣ�
| ���� | �۵�(��) | �е�(��) | �ܶ�(g·cm��3) |
| �Ҵ� | ��117.0 | 78.0 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | ��83.6 | 77.5 | 0.90 |
| Ũ����(98%) | — | 338.0 | 1.84 |
ѧ����ʵ������ȡ������������Ҫ�������£�
����30 mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ��(װ������������)����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10
min��
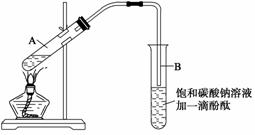
�۴��Թ�B�ռ���һ�����IJ����ֹͣ���ȣ���ȥ�Թ�B��������Ȼ���ô��ֲ㣻
�ܷ�������������㡢ϴ�ӡ����
�������ĿҪ��ش��������⣺
(1)���Ƹû����Һ����Ҫ��������Ϊ
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
д����ȡ���������Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
(2)����ʵ���б���̼������Һ��������(����
ĸ)________________________________________________________________________��
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
(3)���������ҪС����ȼ��Ȳ���������Ҫ������
________________________________________________________________________
________________________________________________________________________��
(4)���������������Ϊ�˸�������������ѡ�õĸ����Ϊ(����ĸ)______��
A��P2O5 B����ˮNa2SO4
C����ʯ�� D��NaOH����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com