

 ClO-+H2��������������Һˮ���Լ��ԣ�ClO-+H2O?HClO+OH-���ʴ�Ϊ��Cl-+H2O
ClO-+H2��������������Һˮ���Լ��ԣ�ClO-+H2O?HClO+OH-���ʴ�Ϊ��Cl-+H2O ClO-+H2�������ڣ�ClO-+H2O?HClO+OH-��
ClO-+H2�������ڣ�ClO-+H2O?HClO+OH-�� 2H2O+2Cl2��+O2����
2H2O+2Cl2��+O2���� 2H2O+2Cl2��+O2����
2H2O+2Cl2��+O2���� =0.05mol/L��100��ʱKw=1x10-12��c��H+��=
=0.05mol/L��100��ʱKw=1x10-12��c��H+��= =2×10-11mol/L��PH=-lg2×10-11mol/L=10.7���ʴ�Ϊ��10.7��
=2×10-11mol/L��PH=-lg2×10-11mol/L=10.7���ʴ�Ϊ��10.7��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?����ģ�⣩NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��2013?����ģ�⣩NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣�NaClO��KAl(SO4)2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��1��NaClO��ҺpH>7��ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ�� ��
��2������NaClO�������Ʋ⣬��ֽ���м���NaClO��Һ��Ŀ���� ��
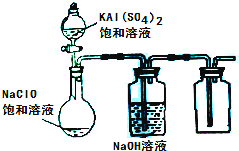
��3��ijС��ͬѧ����ͼ��ʾװ��̽������NaClO��KAl(SO4)2��Һ��Ϸ�Ӧ��ʵ�顣
�� ��������ƿ�м��뱥��KAl(SO4)2��Һ�����������İ�ɫ��״���� ����Ӧ�����ӷ���ʽ�� ��
�� ����ƿ�еĻ��Һ�����������£�������ƿ���л���ɫ�����������ַ�Ӧ����ƿ��������ʹ�������ľ����ȼ��д���ڹ����»��Һ�з�Ӧ�Ļ�ѧ����ʽ��
��
��4������V1 mL 0.1mol/L KAl(SO4)2��Һ��V2mL 0.1mol/LBa(OH)2��Һ��ϡ��������������ʵ������ʱ��V1:V2 = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ��ɽ�и�����ѧģ���Ծ����ˣ� ���ͣ�ʵ����
��16�֣�NaClO��KAl(SO4)2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��1��NaClO��ҺpH>7��ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ�� ��
��2������NaClO�������Ʋ⣬��ֽ���м���NaClO��Һ��Ŀ���� ��
��3��ijС��ͬѧ����ͼ��ʾװ��̽������NaClO��KAl(SO4)2��Һ��Ϸ�Ӧ��ʵ�顣
�ٴ�������ƿ�м��뱥��KAl(SO4)2��Һ�����������İ�ɫ��״��������Ӧ�����ӷ���ʽ�� ��
�ڽ���ƿ�еĻ��Һ�����������£�������ƿ���л���ɫ�����������ַ�Ӧ����ƿ��������ʹ�������ľ����ȼ��д���ڹ����»��Һ�з�Ӧ�Ļ�ѧ����ʽ��
��
��4������V1 mL 0.1mol/L KAl(SO4)2��Һ��V2mL 0.1mol/LBa(OH)2��Һ��ϡ��������������ʵ������ʱ��V1:V2 = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�����и�����һ������Կ������ۻ�ѧ�Ծ��������棩 ���ͣ������
NaClO��KAl(SO4)2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��1����ҵ�Ͽ����Ȼ���Ϊԭ�ϣ�ͨ�����ķ����Ƶ�NaClO����ҵ����ȡNaClO�����ӷ�Ӧ����ʽΪ
�������ҺPH 7������ڡ�С�ڡ����ڣ�����ԭ���� �������ӷ���ʽ��ʾ��
��2��KAl(SO4)2��Һ�������غ��ʽΪ
��3��ijС��ͬѧ����ͼ��ʾװ��̽������NaClO��KAl(SO4)2��Һ��Ϸ�Ӧ��ʵ�顣

�ٴ�������ƿ�м��뱥��KAl(SO4)2��Һ�����������İ�ɫ��״��������ʱ��Ӧ�����ӷ���ʽΪ
��
�ڽ���ƿ�еĻ��Һ�����������£�������ƿ���л���ɫ�����������ַ�Ӧ����ƿ���ռ���һ����ɫ��ζ�����塣д���ڹ������»��Һ�з�Ӧ�Ļ�ѧ����ʽ�� ��
��4��������Һ©���е�KAl(SO4)2��Һ������������泥�һ�ָ���:(NH4)2SO4��FeSO4����Һ,�������䡣��Һ©����������ƿ�е��������������������Һ���۲쵽��ƿ���к��ɫ��������������û�й۲쵽����ɫ�����������ʱ��ƿ�з�����������ԭ��Ӧ�����ӷ���ʽΪ
��
��5��ȡ100mL 0.1mol/L Ba(OH)2��Һ����������μ���ͬŨ�ȵ�KHSO4��Һ��Ba2+ǡ����ȫ��������ʱ��Һ��PHֵΪ ����������Һ���ʱ������仯����Ϻ���Һ���¶�Ϊ100�棬100��ʱKw=1x10-12)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ��ɽ�и�����ѧģ���Ծ����ˣ� ���ͣ�ʵ����
��16�֣�NaClO��KAl(SO4)2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��1��NaClO��ҺpH>7��ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ�� ��
��2������NaClO�������Ʋ⣬��ֽ���м���NaClO��Һ��Ŀ���� ��
��3��ijС��ͬѧ����ͼ��ʾװ��̽������NaClO��KAl(SO4)2��Һ��Ϸ�Ӧ��ʵ�顣

�� ��������ƿ�м��뱥��KAl(SO4)2��Һ�����������İ�ɫ��״���� ����Ӧ�����ӷ���ʽ�� ��
�� ����ƿ�еĻ��Һ�����������£�������ƿ���л���ɫ�����������ַ�Ӧ����ƿ��������ʹ�������ľ����ȼ��д���ڹ����»��Һ�з�Ӧ�Ļ�ѧ����ʽ��
��
��4������V1 mL 0.1mol/L KAl(SO4)2��Һ��V2mL 0.1mol/LBa(OH)2��Һ��ϡ��������������ʵ������ʱ��V1:V2 = ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com