��������1�������ָ����Һ�л�����״̬���ܵ���Ļ��������Һ�к�����״̬�¶����ܵ���Ļ��������ڷǵ���ʣ������ǵ���ʰ������ǽ��������������л�����Ҵ��������ǵȣ��������ȣ�
��2��K
+���Ը�����ء�����صĵ��룬��n=
���������ء�����ص����ʵ��������ݼ������غ��֪n��K
+��=n��KMnO
4��+2n��K
2SO
4�����ٸ������ʵ���Ũ�ȶ��������������ʵ���Ũ�ȣ�
��3���ٸ�������ƿֻ��һ���̶��߲���������ƿ�ij��ù����ѡ��
�ڸ���n=CV ��m=nM�����㣻
�ݸ��ݶ��ݵIJ�����������
��������ƿ��ʹ�õ�ע��������������
�߷������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
�����жϣ�
���
�⣺��1��A�����Ǻ������Ƿ��ӣ���ˮ��Һ�У����ܵ��룬ֻ���ڷ��ӣ����ܵ��磬�Ƿǵ���ʣ���A��ȷ��
B������أ��ܵ������������Ӻͼ����ӣ��ܵ��磬�ǻ�����ǵ���ʣ���B����
C������������Σ�����ˮ��Һ�к�����״̬�µ���������ƶ������Ӷ����磬�ǵ���ʣ���B����
D��������������ˮ��Һ�л�������״̬�¾��ܹ����磬�ǵ���ʣ���D����
�ʴ�Ϊ��A��
��2��������ص����ʵ���Ϊn��KMnO
4��=
=
=
mol������ص����ʵ���Ϊn��K
2SO
4��=
=
mol��
n��K
+��=n��KMnO
4��+2n��K
2SO
4��=
mol+2��
mol������c��K
+��=
mol/L��
�ʴ�Ϊ��
mol/L��
��3������������ƿֻ��һ���̶��ߣ���ֻ�����ƺ��������Ӧ���������Һ������ƿ���õĹ����100ml��250ml��500ml��1000ml��������480ml������ƿ����Ӧѡ��500mL������ƿ���ʴ�Ϊ��500��
������ʹ��500ml����ƿ�������Ƴ�����500ml��Һ����������ʵ����ʵ���n=CV=0.5L��0.2mol/L=0.1mol������m=nM=0.1mol��74.5g/mol=7.5g��
�ʴ�Ϊ��7.5��
�ݶ���ʱ��������ƿ��עˮ����Һ����̶���1��2cmʱ�����ý�ͷ�ι���μ��룬����Һ����̶������м��ɣ��ʴ�Ϊ����ͷ�ιܣ�
������ƿֻ����������һ��Ũ�ȵ���Һ�������ܽ���塢ϡ��Ũ��Һ�ʹ�����Һ�����ƺ���Һ���뼰ʱת�Ƴ����ʴ�Ϊ�����ܣ�
��A������ʱ�۲�Һ�����ӣ��ᵼ����Һ���ƫ��Ũ��ƫ�ͣ���Aѡ��
B������ʱ�۲�Һ�温�ӣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ���B��ѡ��
C��������KCl��Һ�������ձ��У��ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ���Cѡ��
D��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬��D��ѡ��
E������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�NaCl��Һ��δϴ���������ʵ�������Һ�������Ӱ�죬������ҺŨ����Ӱ�죬��E��ѡ��
F������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶����������ģ�δ���κδ�������ȷ�ģ������Ũ�����Ӱ�죬��F��ѡ��
G������ҩƷʱ�������⣬��������������m
��=m
��+m
�������������ҩƷ����������Ũ��ƫ�ߣ���G��ѡ��
��ѡAC��




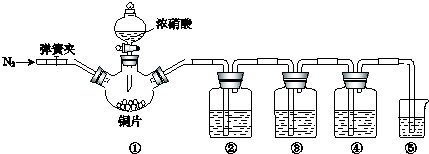
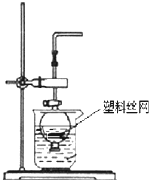 �÷�Һ©�����ձ�����װ���һ���ģ����濪���á������ͣ�����巢��װ�ã���ͼ��ʾ�����ô�װ���Ʊ��������У�������
�÷�Һ©�����ձ�����װ���һ���ģ����濪���á������ͣ�����巢��װ�ã���ͼ��ʾ�����ô�װ���Ʊ��������У�������