



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������������� |
| B����������Na+Ũ�Ȳ��� |
| C��ˮ�Ȳ���������Ҳ���ǻ�ԭ�� |
| D����Ӧ�����ǰѻ�ѧ��ת���ɵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ũ�� ʱ��/s | 0 | 20 | 40 | 60 | 80 | 100 |
| c��N204��/mol?L-1 | 0.1 | c1 | 0.05 | c3 | c3 | c3 |
| c��N02��/mol?L-1 | 0 | 0.06 | c2 | 0.12 | 0.12 | 0.12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ɭ����ҿ��ҷ����ƻ�����̬���� |
| B�������ų�����β�� |
| C�������ж�����̼�ĺ������� |
| D������ʯȼ�ϵĴ���ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ʵ����� | ʵ������ | �� �� |
| A | ������Fe��NO3��2������ˮ�ܽ⣬�μ�ϡH2SO4�ữ���ٵμ�KSCN��Һ | ��Һ��ɺ�ɫ | Fe��NO3��2�����ѱ��� |
| B | ������ij��ɫ����ͨ�����ʯ��ˮ�� | ���ְ�ɫ���� | ������һ����CO2 |
| C | �ֱ�ⶨ������0.1mol?L-1Na2SiO3��Һ��Na2CO3��Һ��pH | pH��Na2SiO3��Na2CO3 | �ǽ����ԣ�Si��C |
| D | ��Ũ�Ⱦ�Ϊ0.1mol?L-1NaCl��NaI�����Һ�У��μ�����AgNO3��Һ | ���ֻ�ɫ���� | Ksp��AgCl����Ksp��AgI�� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
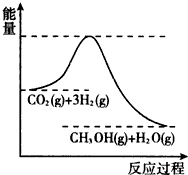 ��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�����÷�Ӧ�������仯��ͼ��ʾ��
��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�����÷�Ӧ�������仯��ͼ��ʾ��| ��t/min | ��0 | ��2 | ��5 | ��10 | ��15 |
| ��n��CO2��/mol | ��1 | ��0.75 | ��0.5 | ��0.25 | ��0.25 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʯī�Ƚ��ʯ�ȶ� |
| B�����ʯ��ʯī�ȶ� |
| C��1 molʯī��1 mol ���ʯ���������� |
| D��1 mol���ʯ��1 mol ʯī���������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com