


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
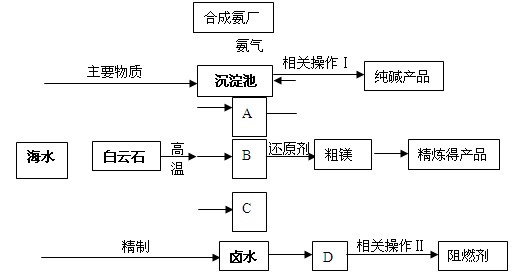
 ���ͣ������ա�
���ͣ������ա��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Һ̬�����Ƶ��� | B��NO��O2����NO2 |
| C����������������������NO | D����ҵ�ϳɰ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ϳɰ����������н�NH3Һ�����룬�ɼӿ�����Ӧ���ʣ����N2��H2��ת���� |
| B�����Ṥҵ�У��ڽӴ��Ұ�װ�Ƚ�������Ϊ������SO3ת��ΪH2SO4ʱ�ų������� |
| C����ⱥ��ʳ��ˮ���ռ�������ӽ���Ĥ�����ɷ�ֹ�����Ҳ�����Cl2���������� |
| D����ͳ�Ĺ����ι�ҵΪ�����ṩ�˴����Ľ����Ȳ��ϡ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ά | B�������оƬ | C��ʯӢ�ӱ� | D��������� |
�鿴�𰸺ͽ���>>
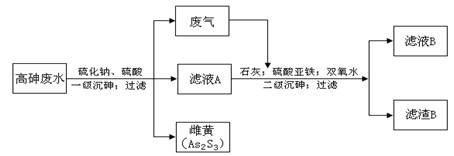
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 -1
-1 �أ��õ�ZnO 2.43g�ͱ�״����CO20.224l����ʽ̼��п�Ļ�ѧʽ
�أ��õ�ZnO 2.43g�ͱ�״����CO20.224l����ʽ̼��п�Ļ�ѧʽ�鿴�𰸺ͽ���>>
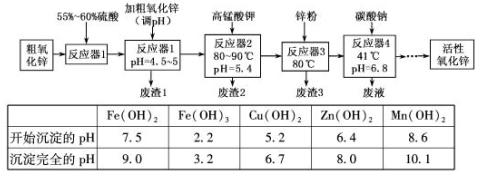
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
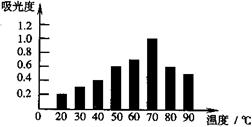
| A��20��ʱ�������к�ɫ�غ������ | |
| B�������Խ�����к�ɫ�غ���Խ�� | C����ɫ�ؿ����ڽϸ��¶��²��ȶ� |
| D���¶�Խ�ߣ���ȡҺ�к�ɫ�غ���Խ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com