

| 3 |
| 2 |
| 1 |
| 2 |
| 0.75mol |
| 1.5mol |
| c2(NH3) |
| c(N2)?c3(H2) |
| c2(NH3) |
| c(N2)?c3(H2) |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� | ���� | ���� | �� |
| �۵㣨�棩 | -210.01 | -252.77 | -77.74 |
| �е㣨�棩 | -195.79 | -259.23 | -33.42 |
| [NH3]2 |
| [N2][H2]3 |
| [NH3]2 |
| [N2][H2]3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| c2(NH3) |
| c(N2)?c3(H2) |
| c2(NH3) |
| c(N2)?c3(H2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| O | 2- 3 |
| O | - 3 |
| H | + 4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ������ѧ�ڿ�ѧˮƽ��⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��14�֣���ҵ�Ϻϳɰ�����һ�������½������·�Ӧ��N2(g) + 3H2(g) �P 2NH3(g)���䲿�ֹ����������£�
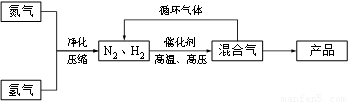
�ش��������⣺
(1) ��֪�� N2(g)
+ O2(g)  2NO(g) ��H
2NO(g) ��H +180.5kJ/mol
+180.5kJ/mol
4NH3(g)
+ 5O2(g)  4NO(g) + 6H2O(g) ��H
4NO(g) + 6H2O(g) ��H −905kJ/mol
−905kJ/mol
2H2(g)
+ O2(g)  2H2O(g) ��H
2H2O(g) ��H −483.6kJ/mol
−483.6kJ/mol
��N2(g)
+ 3H2(g) �P 2NH3(g) ��H  ________________��
________________��
(2) �����ҵ�ϣ���һ���¶��£���1.5 mol N2�����6 mol H2����ͨ�뵽���Ϊ1�����ܱ������С�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%������ƽ�ⳣ��Ϊ_______���ı�������������ʹƽ��������Ӧ���������ƽ�ⳣ���������__________��
������ѹǿ ������Ӧ���Ũ�� ��ʹ�ô��� �ܽ����¶�
(3) �ϳɰ���Ӧ��ƽ�ⳣ����С�������ڹ�ҵ�ϲ�ȡ����ѭ�������̡�����Ӧ��ͨ�����ͻ��������¶ȶ�ʹ����������������ַ������ʵķ�����ԭ�����������з����е�________�����ţ�����������__________��
�ٹ��� ������ ������ ����ȡ
(4) �������������������Ͱ����Ĺܵ��Ƿ�©�������©������а��̣��ɷ�Ϊ�Ȼ�泥����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ__________��
(5) ����ó�������ˮ�����ʵ���Ũ��Ϊ20 mol/L��ʵ����������80 mLŨ��Ϊ5 mol/L�İ�ˮʱ����ȡ20 mol/L�İ�ˮ__________mL����100 mL������ƿ��������ð�ˮ�� ��������ͬ���������ʱ����Һ�����ԣ���������pH__________
��������ͬ���������ʱ����Һ�����ԣ���������pH__________ ������ڡ�����С�ڡ����ڡ�����
������ڡ�����С�ڡ����ڡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�����и����ڶ��ε��п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���ҵ�Ϻϳɰ�����һ�������½������·�Ӧ��
N2��g����3H2��g�� 2NH3��g���ش��������⣺
2NH3��g���ش��������⣺
��1����֪��N2��g����O2��g����2NO��g������H����180��5kJ��mol
4NH3��g����5O2��g����4NO��g����6H2O��g���� ��H����905kJ��mol
2H2��g����O2��g����2H2O��g���� ��H����483��6kJ��mol
��N2��g����3H2��g�� 2NH3��g���ġ�H��_______________��
2NH3��g���ġ�H��_______________��
��2����NH4��2SO3��NH4HSO4�����ֻ���ԭ�ϡ�
�ٶ��ڣ�NH4��2SO3��Һ��ijͬѧд�������µ���ȷ��ϵʽ��
2[c�� ����c��
����c�� ����c��H2SO3��]��c��
����c��H2SO3��]��c�� ����c��NH3��H2O��
����c��NH3��H2O��
��ͬѧ�������ǣ�_________________________________________
��д��NH4HSO4��Һ������Ũ���ɴ�С��˳��______________________��
��3�����顪������ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20����30����KOH��Һ�����ü��顪������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飨����a��b��Ϊ̼������

��ZnƬ�Ϸ����ĵ缫��Ӧʽ��_____________________��
��a�缫�ĵ缫��Ӧʽ_______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com