
J”¢L”¢M”¢R”¢TŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬J”¢RŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĻĀ±ķ£»JŌŖĖŲ×īµĶøŗ»ÆŗĻ¼ŪµÄ¾ų¶ŌÖµÓėĘäŌ×Ó×īĶā²ćµē×ÓŹżĻąµČ
|
|
|
J |
|
|
|
|
|
|
|
R |
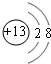
MµÄĘųĢ¬Ō×ÓÖšøöŹ§Č„1~ 4øöµē×ÓĖłŠčÄÜĮæ£ØµēĄėÄÜ£©ČēĻĀ±ķĖłŹ¾£¬
|
|
I1 |
I2 |
I3 |
I4 |
”” |
|
µēĄėÄÜ(kJ/mol) |
578 |
1817 |
2745 |
11578 |
”” |
£Ø1£©MµÄµē×ÓÅŲ¼Ź½ĪŖ________£»ŌŖĖŲTŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ________”£
£Ø2£©JŗĶĒāÄÜŠĪ³É¶ąÖÖ»ÆŗĻĪļ£¬ĘäÖŠ·Ö×Ó³ÉÖ±ĻߊĶµÄ£¬ĒŅĻą¶Ō·Ö×ÓÖŹĮæ×īŠ”µÄĪļÖŹµÄ½į¹¹Ź½ĪŖ________”£
£Ø3£©MŗĶTŠĪ³ÉµÄ»ÆŗĻĪļŌŚ³±ŹŖµÄæÕĘųÖŠĆ°°×Īķ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________________”£
£Ø4£©ÓÉJ”¢RŠĪ³ÉµÄŅŗĢ¬»ÆŗĻĪļJR2 0.2 molŌŚO2ÖŠĶźČ«Č¼ÉÕ£¬Éś³ÉĮ½ÖÖĘųĢ¬Ńõ»ÆĪļ£¬298 KŹ±·Å³öČČĮæ215 kJ”£øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ________”£
£Ø5£©ÄÜŌ“²ÄĮĻŅŃ³ÉĪŖµ±½ńæĘѧъ¾æµÄČČµć”£ĒāĘų×÷ĪŖŅ»ÖÖĒå½ąÄÜŌ“£¬±ŲŠė½ā¾öĖüµÄ“¢“ęĪŹĢā£¬C60æÉÓĆ×÷“¢Ēā²ÄĮĻ”£¼ĢC60ŗó£¬æĘѧ¼ŅÓÖŗĻ³ÉĮĖSi60”¢N60£¬ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ_______£ØĢīŠņŗÅ£©”£
a. C60”¢Si60”¢N60¶¼ŹōÓŚŠĀŠĶ»ÆŗĻĪļ
b. C60”¢Si60”¢N60»„ĪŖĶ¬·ÖŅģ¹¹Ģå
c. ŅŃÖŖN60½į¹¹ÓėC60ĻąĖĘ£¬ÓÉÓŚN-N¼üÄÜŠ”ÓŚN”ŌN£¬¹ŹN60µÄĪČ¶ØŠŌČõÓŚN2
d. ŅŃÖŖ½šøÕŹÆÖŠC£C¼ü³¤154pm£¬C60ÖŠC£C¼ü³¤145~140pm£¬¹ŹC60ČŪµćøßÓŚ½šøÕŹÆ
£Ø1£©1s22s22p63s23p1 µŚČżÖÜĘŚ¢÷A×å
£Ø2£©H”ŖC”ŌC”ŖH
£Ø3£©AlCl3£«3H2O”śAl(OH)3£«3HCl”ü
£Ø4£©CS2(l)£«3O2(g) =CO2(g)£«2SO2(g) ”÷H=-1075 kJ/mol£Ø2·Ö£©
£Ø5£©c£Ø2·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗJŌŖĖŲ×īµĶøŗ»ÆŗĻ¼ŪµÄ¾ų¶ŌÖµÓėĘäŌ×Ó×īĶā²ćµē×ÓŹżĻąµČ£¬ĖµĆ÷JµÄ×īµĶ»ÆŗĻ¼ŪÓė×īøß»ÆŗĻ¼Ū¾ų¶ŌÖµĻąµČ£¬ŌņJ×īĶā²ćµē×ÓŹżĪŖ4£¬øł¾ŻJ”¢R¶¼ĪŖ¶ĢÖÜĘŚŌŖĖŲæÉÖŖJĪŖCŌŖĖŲ£¬ŌņRÓ¦ĪŖSŌŖĖŲ”£
£Ø1£©“ÓMµÄµēĄėÄÜĄ“æ“Ē°Čżøöµē×ÓÖšøöŹ§Č„ĖłŠčÄÜĮæI1 ”¢I2 ”¢I3Ļą²ī²»“󣬶ųI4 Ļą²ī·Ē³£“ó£¬ÓÉ“ĖæÉŅŌĶĘ²āĒ°3øöµē×ÓŌŚĶ¬Ņ»×īĶā²ć£¬µŚ4øöµē×ÓŌŚ“ĪĶā²ć£¬ÓÉ“ĖæÉĶĘ²āMĪŖAlŌŖĖŲ£¬ĖłŅŌĘäµē×ÓÅŲ¼ĪŖ1s22s22p63s23p1 £»ŌŖĖŲTŹĒŌŚRŗó±ßµÄÖ÷×åŌŖĖŲ£¬RŹĒSŌŖĖŲ£¬ŌņTŹĒClŌŖĖŲ£¬Ī»ÖĆĪŖµŚČżÖÜĘŚ¢÷A×唣
£Ø2£©JĪŖCŌŖĖŲ£¬ĖłŅŌŗĶĒāÄÜŠĪ³É¶ąÖÖ»ÆŗĻĪļĪŖÓŠ»ś»ÆŗĻĪļ£¬ĘäÖŠ³ŹÖ±ĻߊĶµÄŗ¬ÓŠC”ŌCµÄČ²Ąą£¬Ļą¶Ō·Ö×Ó×īŠ”µÄČ²ĄąĪŖŅŅČ²£¬Ęä½į¹¹Ź½ĪŖH”ŖC”ŌC”ŖH”£
£Ø3£©MŗĶTŠĪ³ÉµÄ»ÆŗĻĪļĪŖAlCl3£¬ŹĒŅ»ÖÖĒæĖįČõ¼īŃĪ£¬ŌŚ³±ŹŖ»·¾³ĻĀČŻŅ×Ė®½ā²śÉśŅ×»Ó·¢µÄHCl£¬Č»ŗóŠĪ³ÉĖįĪķ£¬ĖłŅŌ·“Ó¦·½³ĢŹ½ĪŖAlCl3£«3H2O=Al(OH)3£«3HCl”ü”£
£Ø4£©J”¢RŠĪ³ÉµÄŅŗĢ¬»ÆŗĻĪļJR2 ĪŖCS2£¬ĘäĶźČ«Č¼ÉÕÉś³ÉCO2”¢SO2£¬0.2mol·Å³öČČĮæ215kJ£¬ĖłŅŌ1molŹ±Č¼ÉշųöČČĮæ1075 kJ£¬ĖłŅŌČČ»Æѧ·½³ĢĪŖCS2(l)£«3O2(g) =CO2(g)£«2SO2(g) ”÷H=-1075 kJ/mol”£
£Ø5£©C60”¢Si60”¢N60¶¼ŹĒµ„ÖŹ£¬aŃ”Ļī“ķĪó£»C60”¢Si60”¢N60×é³ÉµÄŌŖĖŲ²»Ķ¬£¬²»ŹōÓŚĶ¬·ÖŅģ¹¹Ģ壬bĻī“ķĪó£»ÓÉÓŚN-N¼üÄÜŠ”ÓŚN”ŌN£¬N60½į¹¹ÓėC60ĻąĖĘ£¬ŌņŠĪ³ÉµÄ·Ö×ÓÖŠ¶¼ŹĒµ„¼ü£¬¹ŹN60µÄĪČ¶ØŠŌČõÓŚN2 £¬cÕżČ·£»dĻīÖŠĖäČ»½šøÕŹÆÖŠC£C¼ü³¤ŅŖ³¤ÓŚC60ÖŠC£C¼ü³¤£¬µ«ŹĒ½šøÕŹÆŹĒæÕ¼äĶųד½į¹¹·Ē³£ĪČ¶Ø£¬¶ųC60·Ö×ÓŌņŹĒ×ćĒņÄ£ŠĶ½į¹¹²»¹»ĪČ¶Ø£¬ĖłŅŌ½šøÕŹÆČŪµćøßÓŚC60ČŪµć£¬d“ķĪ󣬹ŹŃ”c”£
æ¼µć£ŗ±¾Ģāæ¼²éµÄŹĒŌŖĖŲÖÜĘŚ±ķŗĶÖÜĘŚĀɵÄÖŖŹ¶”£


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2010?ø£½Ø£©J”¢L”¢M”¢R”¢TŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬J”¢RŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēÓŅ±ķ£»JŌŖĖŲ×īµĶøŗ»ÆŗĻ¼ŪµÄ¾ų¶ŌÖµÓėĘäŌ×Ó×īĶā²ćµē×ÓŹżĻąµČ£»MŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲ£®
£Ø2010?ø£½Ø£©J”¢L”¢M”¢R”¢TŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬J”¢RŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēÓŅ±ķ£»JŌŖĖŲ×īµĶøŗ»ÆŗĻ¼ŪµÄ¾ų¶ŌÖµÓėĘäŌ×Ó×īĶā²ćµē×ÓŹżĻąµČ£»MŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲ£®

 Al£ØOH£©3+3HCl
Al£ØOH£©3+3HCl Al£ØOH£©3+3HCl
Al£ØOH£©3+3HCl| Ń”Ļī | a | b | c | d |
| x | ĪĀ¶Č | ĪĀ¶Č | ¼ÓČėN2µÄĪļÖŹµÄĮæ | ¼ÓČė¼×µÄĪļÖŹµÄĮæ |
| y | ¼×µÄĪļÖŹµÄĮæ | Ę½ŗā³£ŹżK | ¼×µÄ×Ŗ»ÆĀŹ | Éś³ÉĪļĪļÖŹµÄĮæ×ÜŗĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 J”¢L”¢M”¢R”¢TŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬J”¢RŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēÓŅ±ķ£»JŌŖĖŲ×īµĶøŗ»ÆŗĻ¼ŪµÄ¾ų¶ŌÖµÓėĘäŌ×Ó×īĶā²ćµē×ÓŹżĻąµČ£»MŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲ£®
J”¢L”¢M”¢R”¢TŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬J”¢RŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēÓŅ±ķ£»JŌŖĖŲ×īµĶøŗ»ÆŗĻ¼ŪµÄ¾ų¶ŌÖµÓėĘäŌ×Ó×īĶā²ćµē×ÓŹżĻąµČ£»MŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲ£®

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 J”¢L”¢M”¢R”¢TŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬J”¢RŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČē±ķ£»JŌŖĖŲ×īµĶøŗ»ÆŗĻ¼ŪµÄ¾ų¶ŌÖµÓėĘäŌ×Ó×īĶā²ćµē×ÓŹżĻąµČ£»MŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲ£®
J”¢L”¢M”¢R”¢TŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬J”¢RŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČē±ķ£»JŌŖĖŲ×īµĶøŗ»ÆŗĻ¼ŪµÄ¾ų¶ŌÖµÓėĘäŌ×Ó×īĶā²ćµē×ÓŹżĻąµČ£»MŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| J | ||||
| M | R |

| Ń”Ļī | a | b | c | d |
| x | ĪĀ¶Č | ¼ÓČėH2µÄĪļÖŹµÄĮæ | ¼ÓČėH2µÄĪļÖŹµÄĮæ | ¼ÓČė¼×µÄĪļÖŹµÄĮæ |
| y | ¼×µÄĪļÖŹµÄĮæ | Ę½ŗā³£ŹżK | ¼×µÄ×Ŗ»ÆĀŹ | Éś³ÉĪļĪļÖŹµÄĮæ×ÜŗĶ |
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com