
| A�� | c��H+��•c��OH-��=10-14 | B�� | c��Na+��+c��K+��=c��HA-��+2c��A2-�� | ||
| C�� | c��Na+����c��K+�� | D�� | c��Na+��+c��K+��=0.05mol•L-1 |
���� A��ˮ�����ӻ��������¶��йأ��¶�Խ�ߣ�ˮ�����ӻ�����Խ��
B��������Һ�еĵ������жϣ�
C����0.1mol/L��NaHA��Һ����εμ�0.1mol/L KOH��Һ����Һ������ʱ��NaHA�����ʵ���Ӧ�����������ص����ʵ�����
D����0.1mol/L��NaHA��Һ����εμ�0.1mol/L KOH��Һ����Һ������ʱ�������Һ�������غ�����жϣ�
��� �⣺A��ˮ�����ӻ��������¶��йأ��¶�Խ�ߣ�ˮ�����ӻ�����Խ���¶�δ֪������ˮ�����ӻ�������һ��Ϊ10-14����A����
B����Һ�ʵ����ԣ���Һ�������������������ȣ����ݵ���غ�ɵã�c��Na+��+c��K+��=c��HA-��+2c��A2-������B��ȷ��
C��NaHA��Һ�����ԣ�Na2A��Һ�ʼ��ԣ���0.1mol/L��NaHA��Һ����εμ�0.1mol/L KOH��Һ����Һ������ʱ��NaHA�����ʵ���Ӧ�����������ص����ʵ���������ͬһ�����Һ��c��Na+����c��K+������C��ȷ��
D��NaHA��Һ�����ԣ�Na2A��Һ�ʼ��ԣ���0.1mol/L��NaHA��Һ����εμ�0.1mol/L KOH��Һ����Һ������ʱ��NaHA�����Ӧ�����������ص����������c��Na+��+c��K+����0.05mol/L����D����
��ѡBC��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ����غ㡢�����غ㼰�ε�ˮ��ԭ���ĺ��弰Ӧ�÷���Ϊ���ؼ�������������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
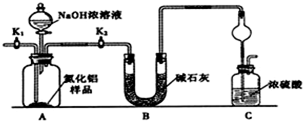
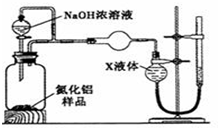
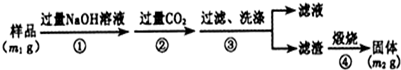
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����ұ���ʹ������漰������ԭ��Ӧ��
����ұ���ʹ������漰������ԭ��Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2��MgO | B�� | Fe3O4��H2O | C�� | FeCl3��CuCl2 | D�� | MnO2��Al2O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | MnO2��ŨHCl���ã�MnO2+4HCl��Ũ��$\stackrel{��}{��}$MnCl2+Cl2��+2H2O | |
| B�� | KMnO4+16HCl��Ũ����2KCl+2MnCl2��+5Cl2��+8H2O | |
| C�� | KClO3+6HCl��Ũ����KCl+3Cl2��+3H2O | |
| D�� | Ca��ClO��2+4HCl��Ũ����CaCl2+2Cl2��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
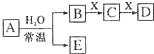 �ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E��X ����������ͼת����ϵ������������ͷ�Ӧ������ȥ����
�ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E��X ����������ͼת����ϵ������������ͷ�Ӧ������ȥ����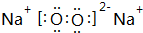 ����NaHCO3�ֽ���������壨ˮ�����Ͷ�����̼����������A������յõ�����ף���������500mL 1mol/L�������У�������ɫ������壬��Һ�����ԣ���A�����ʵ���Ϊ0.25mol����������ڱ�״���µ����Ϊ2.8L��������ˮ�����IJ���������������ܽ⣩��
����NaHCO3�ֽ���������壨ˮ�����Ͷ�����̼����������A������յõ�����ף���������500mL 1mol/L�������У�������ɫ������壬��Һ�����ԣ���A�����ʵ���Ϊ0.25mol����������ڱ�״���µ����Ϊ2.8L��������ˮ�����IJ���������������ܽ⣩���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com