
��18�֣�Ϊ��֤������Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г������� A�м���װ�����ԣ��������Ѽ��飩ʵ��������£�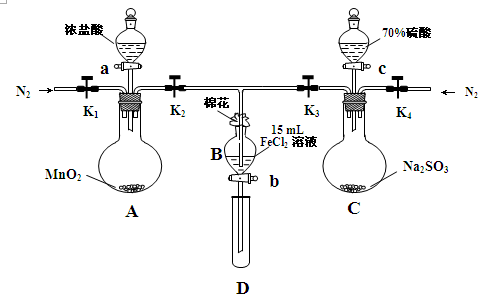
��.���ɼ�K1��K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
��.����a���μ�һ������Ũ���ᣬ��A���ȡ�
��.��B����Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2��
��.����b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
��.���ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3��
��.�����Թ�D���ظ����̢�������B��Һ�е����ӡ�
��1�����̢��Ŀ����_________________________________________________��
��2�����н������ҺΪ______________________��
��3��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ���� ��
��5����˵��������Fe3+��SO2�����ӷ���ʽ��______________________________________��
��6���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬���ǵļ����һ���ܹ�֤��������Cl2��Fe3+��SO2���� ����ס����ҡ�����������
| | ���̢� B��Һ�к��е����� | ���̢� B��Һ�к��е����� |
| �� | ��Fe3+��Fe2+ | ��SO42- |
| �� | ����Fe3+����Fe2+ | ��SO42- |
| �� | ��Fe3+��Fe2+ | ��Fe2+ |
 FeSO3��s����ī��ɫ��
FeSO3��s����ī��ɫ��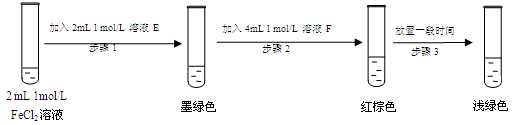
��1���ų�������1�֣� ��2��NaOH��Һ ��3��MnO2 + 4HCl(Ũ) ��MnCl2+Cl2��+ 2H2O
��4��70%H2SO4��c(H+)�� ��5��2Fe3+ + SO2 + 2H2O��2Fe2+ + SO42-+4H+ ��6���ҡ���
��7���� Na2SO3��Һ ��FeCl3��Һ
��Fe3+ ���� SO32-��c(SO32-)��С��ʹƽ��Fe2+(aq)��SO32-(aq) FeSO3(s)�����ƶ�����Һ��ɫ�ɺ���ɫ��Ϊdz��ɫ����3�֣�
FeSO3(s)�����ƶ�����Һ��ɫ�ɺ���ɫ��Ϊdz��ɫ����3�֣�
���������������1����K1��K4���ر�K5��K6��ͨ��һ��ʱ��N2��Ŀ�����ų�װ���е�������
��2�����������������ж������н���NaOH��Һ�����ն�����������������ֹ��Ⱦ������
��3��A�з�������������Ũ����ķ�Ӧ�������Ȼ��̡�������ˮ���÷�ӦΪMnO2 + 4HCl(Ũ) ��MnCl2+Cl2��+ 2H2O��
��4����������Ũ��Խ��Ӧ����Խ�죬��70%�������е�������Ũ�ȱ�98%�������е�������Ũ�ȴ����Է�Ӧ���ʿ졣
��5���������ܰѶ������������������ᣬ��Ӧ�����ӷ���ʽΪ2Fe3+ + SO2 + 2H2O��2Fe2+ + SO42-+4H+��
��6�����е�һ�Σ�˵���������㣬���������Դ��������ӣ��ڶ�������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ���������е�һ����Fe3+����Fe2+���������������Դ��������ӣ��ڶ������������ӣ�˵���������������������ӵķ�Ӧ���������������Ӵ��ڶ������ʴ�Ϊ���ҡ�����
��7����E���Ȼ�������Һ��Ӧ����ī��ɫ��˵����Ӧ�����������������ɣ����EӦ����Na2SO3��Һ������FeSO3��ī��ɫ����FeCl3����ɫ���Ļ�Ϻ���Һ�Ժ���ɫ��֪��FӦ����FeCl3��Һ��
�������Ӿ��������ԣ����Fe3+ ���� SO32-������c(SO32-)��С��ʹƽ��Fe2+(aq)��SO32-(aq) FeSO3(s)�����ƶ�����Һ��ɫ�ɺ���ɫ��Ϊdz��ɫ��
FeSO3(s)�����ƶ�����Һ��ɫ�ɺ���ɫ��Ϊdz��ɫ��
���㣺��������ʵ�鷽������Ƽ�������ԭ��Ӧ��ƽ���ƶ�ԭ��Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ������Ȼ������Ҫ��������������Ҫ�ɷ�ΪAl2O3��������Fe2O3��FeO��SiO2���С���ҵ�����������Ʊ�����ij�ֻ�����Ĺ����������¡�
��1������ҺA�м���Ư��Һ��Ŀ��������������������ҺB�����ԡ�
�ٸù������漰ij������ԭ��Ӧ���£�����ɣ�
��Fe2++��ClO��+�� =��Fe(OH)3��+��C1��+��
�ڼ�����ҺB���Ƿ�����Ԫ�صķ���Ϊ��
��ע���Լ�������
�۽���ҺB�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ ������ţ���
a������������Һ b��������Һ c����ˮ d��������̼
������ҺB�Ʊ��Ȼ��������漰�IJ���Ϊ���ߵμ�Ũ���������Ũ������ȴ�ᾧ�� ����������ƣ���ϴ�ӡ�
��2��SiO2��NaOH�����Ʊ������ƣ��ɲ��õ�װ��Ϊ ������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ����Լ�ȼ�ϵĿ�������������Ҫ���塣
��1��NO2����ˮ���գ����÷�Ӧ6NO2+8NH3 7N2+12H2O��
7N2+12H2O��
Ҳ���Դ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״������______________L��
��2����֪��2SO2(g)+ O2 (g)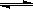 2SO3 (g)������H =��196.6kJ��mol��1
2SO3 (g)������H =��196.6kJ��mol��1
2NO(g)+ O2 (g) 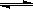 2NO2 (g)������H =��113.0kJ��mol��1
2NO2 (g)������H =��113.0kJ��mol��1
��ӦNO2(g)+ SO2 (g) 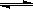 SO3 (g) +NO(g)�ġ�H =______kJ��mol��1
SO3 (g) +NO(g)�ġ�H =______kJ��mol��1
��3��CO�����ںϳɼ״�����Ӧ����ʽΪCO(g)+ 2H2 (g) 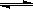 CH3OH (g)���ɼ״��������Լ�ǿ�����������Һ�������ֻ���أ����������������غ�﮵�ص�10����������ʹ��1���³��һ�Ρ��ٶ��ŵ�����У��״���ȫ����������CO2�������������CO32��
CH3OH (g)���ɼ״��������Լ�ǿ�����������Һ�������ֻ���أ����������������غ�﮵�ص�10����������ʹ��1���³��һ�Ρ��ٶ��ŵ�����У��״���ȫ����������CO2�������������CO32��
�ٸõ�ط�Ӧ�������ӷ���ʽΪ____________________________________________��
�ڼ״���____��������Ӧ��������������ڷŵ��������Һ��pH��____________����ͻ����������䣩������16�˼״���������ȫ����,�����ĵ��ܵ��������CuSO4��Һ����������������������������Ϊ80������������״���µ�O2________________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��6�֣���50mL a mol��L-1��������Һ�У�����6.4g Cu��ȫ���ܽ⣬��������Ļ�ԭ����ֻ��NO2��NO������Ӧ����Һ������ˮϡ����100mLʱ���c(NO3-)="3" mol��L-1��
��1����ϡ�ͺ����Һ��pH= ��
��2����a=9�������ɵ�������NO2�����ʵ���Ϊ mol��
��3����������������Ⱦ�ķ���֮һ����NaOH��Һ�������գ���Ӧԭ�����£�
NO2+NO+2NaOH=2NaNO2+H2O 2NO2+2NaOH=NaNO2+NaNO3+H2O
����������NO2��NO�Ļ������ͨ��1mol��L-1��NaOHǡ�ñ����գ���NaOH��Һ�����Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о�С��Ϊ̽��SO2��Fe(NO3)3��Һ�ķ�Ӧ��ʵ�顣���������ͼ��ʾװ�ý���ʵ��.��֪��1.0 mol/L��Fe(NO3)3��Һ��pH��1
��ش�
��1��װ��A����������Ũ�������������Ϊ ��
��2��ʵ��ǰ����N2��Ŀ���� ��
��3��װ��B�в����˰�ɫ��������ɷ���_______��˵��SO2����____�ԡ�
��4������B�в�����ɫ������ԭ��
�۵�1��SO2��Fe3����Ӧ��
�۵�2��������������SO2��NO3-��Ӧ��
�����۵�1��ȷ�������������⣬��Ӧ�۲쵽�������� ��
�ڰ��۵�2��װ��B�з�Ӧ�����ӷ���ʽ�� ��
��������Ϊ���罫װ��B�е�Fe(NO3)3��Һ�滻Ϊ�������������Һ������ͬ�����½���ʵ�飬Ҳ����֤�۵�2�Ƿ���ȷ����ʱӦѡ�������Լ��ǣ�����ţ� ��
| A��1 mol/Lϡ���� |
| B��1.5 mol/L Fe(NO3)2��Һ |
| C��6.0 mol/L NaNO3��Һ��0.2 mol/L����������ϵ���Һ |
| D��3.0 mol/L NaNO3��Һ��0.1mol/L����������ϵ���Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������FeC2O4������������������Ӱ���Լ����͵�ز�����������﮵�������
I��ij��ȤС��Բ��������ķֽ�������ʵ���̽����
��1����֪CO�����Ȼ��٣�PdCl2����Һ��Ӧ���ɺ�ɫ���ٷۡ�
�����������ֽ��������������ͨ��A������ʯ��ˮ����B���Ȼ�����Һ�����۲쵽A�г���ʯ��ˮ����ǣ�B���к�ɫ�������ɡ��ɴ�˵����������к��� ��
��2������Ʒ�����������壨FeC2O4��2H2O������������н������ط������������ͼ��TG��ʾ������������ռԭ��Ʒ�������İٷ�������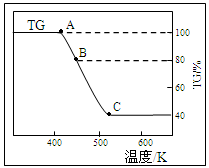
����ȷ��B���Ӧ�������ʵĻ�ѧʽ ��
��д��B C��Ӧ�Ļ�ѧ����ʽ ��
C��Ӧ�Ļ�ѧ����ʽ ��
II��ij����������Ʒ�������ᾧˮ���к����������ᡣ���õζ����ⶨ����Ʒ��FeC2O4�ĺ�����
ʵ�鷽�����£�
�ٽ�ȷ������0.20g����������Ʒ����250 mL��ƿ�ڣ���������2 mol/L��H2SO4��Һ��ʹ��Ʒ�ܽ⣬������70�����ң������ø��������Һ�ζ����յ㡣
����ζ��յ���Һ�м���������Zn�ۺ�����2 mol/L��H2SO4��Һ�����5��8min����KSCN��Һ�ڵ�ΰ��ϼ������Һ��ֱ����Һ����죬�����������һ����ƿ�У���0.02000 mol/L�ĸ�����ر���Һ�ζ�����Һ���յ㣬���ĸ�����ر�Һ6.00 ml��
�Իش��������⣺
��1��������ر�Һ�� �ζ���ʢװ�����ʽ����ʽ������
��2���ڲ�����У��μӸ��������Һʱ�۲쵽����ɫ���������������������ᷴӦ�����ӷ���ʽΪ ��
��3���ڲ�����У����в���������ⶨ���ƫ�ߵ��� ��
| A���ζ�����ʢװ�������ǰδ��ϴ |
| B���ζ������У���ƿ��̫���ң����²���Һ�彦�� |
| C���ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ��� |
| D���ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧʵ����ȤС���ڡ�̽��±�ص��ʵ������ԡ���ϵ��ʵ���з��֣���������ϡ�Ȼ�������Һ�У�����1��2����ˮ������Һ�ʻ�ɫ��
(1)������⣺Fe3����Br2˭�������Ը�ǿ��
(2)������룺
�ټ�ͬѧ��Ϊ�����ԣ�Fe3��>Br2��������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ����Ϊ���� (�ѧʽ����ͬ)��
����ͬѧ��Ϊ�����ԣ�Br2>Fe3����������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ����Ϊ���� ��
(3)���ʵ�鲢��֤����ͬѧΪ��֤��ͬѧ�Ĺ۵㣬ѡ������ijЩ�Լ���Ƴ����ַ�������ʵ�飬��ͨ���۲�ʵ������֤������ͬѧ�Ĺ۵�����ȷ�ġ�
��ѡ�õ��Լ���
a.��̪��Һ b�����Ȼ�̼ c����ˮ�ƾ� d�����軯����Һ
���������б�����д����ͬѧѡ�õ��Լ���ʵ���й۲쵽������(�Լ������)��
| | ѡ���Լ� | ʵ������ |
| ����1 | | |
| ����2 | | |
 ��Br����������Br2����ԭFeBr2��Һ��FeBr2�����ʵ���Ũ��Ϊ ��
��Br����������Br2����ԭFeBr2��Һ��FeBr2�����ʵ���Ũ��Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��֤������Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г������� A�м���װ�����ԣ��������Ѽ��飩��
ʵ����̣�
��.���ɼ�K1~K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
��.����a���μ�һ������Ũ���ᣬ��A���ȡ�
��.��B����Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2��
��.����b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
��.���ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3��
��.�����Թ�D���ظ����̢�������B��Һ�е����ӡ�
��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ���� ��
��3���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬���ǵļ����һ���ܹ�֤��������
Cl2��Fe3+��SO2���� ����ס����ҡ�����������
| | ���̢� B��Һ�к��е����� | ���̢� B��Һ�к��е����� |
| �� | ��Fe3+��Fe2+ | ��SO42- |
| �� | ����Fe3+����Fe2+ | ��SO42- |
| �� | ��Fe3+��Fe2+ | ��Fe2+ |
 FeSO3��s����ī��ɫ��
FeSO3��s����ī��ɫ��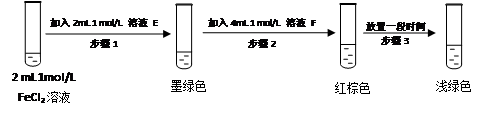
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͬ���ʵ������������ʷֱ����Ũ�ȵ�NaOH��Һ��Ӧ������ϵ�о��������ʣ����ļ��������ǣ� ��
A��Al B��Al(OH)3 C��AlCl3 Al2O3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com