
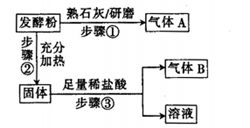
| 实验步骤 |  预期现象结论 预期现象结论 |
| 1.取少量样品溶于稀盐酸后,将溶液分成两份 | |
| 2. _______________________________________ | |
| 3. ________________________________________ | |
| 实验步骤 |  预期现象结论 预期现象结论 |
| 1.取少量样品溶于稀盐酸后,将溶液分成两份 | |
| 2.用光洁无锈的铁丝(1分)蘸取其中一份溶液在酒精灯外焰灼烧,观察火焰颜色(1分)____ | 火焰呈黄色(1分),该发酵粉中含有NaHCO3(1分) |
| 3. 向另外一份溶液中滴加少量(1分)BaCl2溶液(1分)振荡 (少量、1—2滴均可得1分, 试剂BaCl2得分,Ba(NO3)2,Ba(0H)2不得分,多写足量HCl酸化不扣分) | 有白色沉淀生成(1分),结合步骤2的结论假设二(1分)成立 |


科目:高中化学 来源:不详 题型:填空题

查看答案和解析>>
科目:高中化学 来源:不详 题型:单选题
| A.苯中含有苯酚杂质:加入溴水,过滤 |
| B.乙醇中含有乙酸杂质:加入碳酸钠溶液,分液 |
| C.FeCl3溶液中含有CuCl2杂质:加入过量铁粉,过滤 |
| D.CO2中含有HCl杂质:通入饱和NaHCO3溶液,洗气 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:单选题
| A.向饱和FeCl3溶液中滴加过量氨水,可制取Fe(OH)3胶体 |
| B.取少量溶液X,向其中加入适量新制氯水,再加几滴KSCN溶液,溶液变红,说明X溶液中一定含有Fe2+ |
| C.室温下向苯和少量苯酚的混合溶液中加入适量NaOH溶液,振荡.静置后分液,可除去苯中少量苯酚 |
D.已知I3- I2+I-,向盛有KI3溶液的试管中加入适量CCl4,振荡静置后CCl4层显紫色,说明KI3在CCl4中的溶解度比在水中的大 I2+I-,向盛有KI3溶液的试管中加入适量CCl4,振荡静置后CCl4层显紫色,说明KI3在CCl4中的溶解度比在水中的大 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:实验题
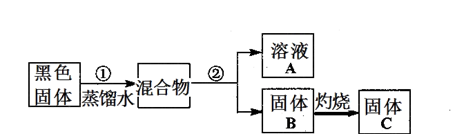
| 实验操作 | 预期现象 | 结论 |
| 步骤1:各取少量溶液A分装a、b、c三支试管,往a试管,__ __________________________ | 有白色沉淀产生 | 说明溶液A含有Cl- |
| 步骤2:往b试管,__________ __________________________ | ______________________ | _______________________ |
| 步骤3:往c试管,__________ __________________________ | 先产生_______________, 后____________________ | 说明溶液A含有Zn2+ |
查看答案和解析>>
科目:高中化学 来源:不详 题型:实验题
| 所加试剂 | 预期现象和结论 |
| 试管A中加足量① ; 试管B中加1%品红溶液; 试管C中加② 。 | 若A中溶液变蓝色,B中溶液不退色,C中溶液变浑浊。则消毒液部分变质; ③ 则消毒液未变质; ④ 则消毒液完全变质。 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:实验题
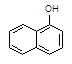 +C2H5OH
+C2H5OH
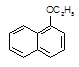 +H2O
+H2O

| 实验目的 | 实验操作 | 预期现象和结论 |
| ①用金属钠检验1-乙氧基萘是否纯净 | 取少量经提纯的产品于试管A中,加入金属钠 | 若 ,则产品纯净; 若 ,则产品不纯。 |
| ②检验经提纯的产品是否含有1-萘酚 | | 若 ,则含有1-萘酚; 若 ,则不含1-萘酚。 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:单选题
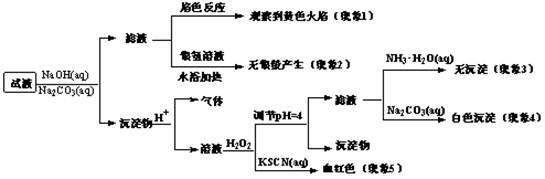
| A.根据现象1可推出该试液中含有Na+ |
| B.根据现象2可推出该试液中并不含有葡萄糖酸根 |
| C.根据现象3和4可推出该试液中含有Ca2+,但没有Mg2+ |
| D.根据现象5可推出该试液中一定含有Fe2+ |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com