
| ʵ���� | ʵ���¶�/�� | c��Na2S2O3��/mol?L-1 | c��H2SO4��/mol?L-1 |
| �� | 25 | 0.1 | 0.1 |
| �� | 25 | 0.1 | 0.2 |
| �� | 50 | 0.2 | 0.1 |
| �� | 50 | 0.1 | 0.1 |
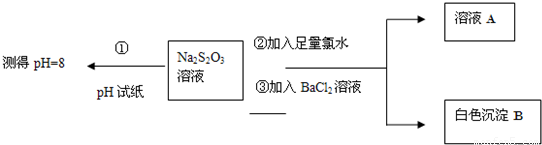


 ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д� ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
��1��CS2��Na2Sˮ��Һһ����ˮ��Һ����ɫ����ɫ________________________��
��2��P2S5����K2Sˮ��Һ��________________��
��3��Na2S2��Һ�м�ϡHCl������ɫ�ij���________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
��2����֪Mg������CO2��ȼ�գ��仯ѧ��Ӧ����ʽΪ��2Mg+CO2![]() 2MgO+C��SO2��Mg�ɷ������Ƶķ�Ӧ����Mg��SO2��ȼ�յIJ��������______________����Ӧ�Ļ�ѧ����ʽΪ__________________��
2MgO+C��SO2��Mg�ɷ������Ƶķ�Ӧ����Mg��SO2��ȼ�յIJ��������______________����Ӧ�Ļ�ѧ����ʽΪ__________________��
��3����֪����ͬ�壬�������ƣ����û�ѧ����ʽ��������ʵ������
��CS2��Na2Sˮ��Һһ����ˮ��Һ����ɫ��Ϊ��ɫ______________��
��P2S5����K2Sˮ��Һ��_____________________��
��Na2S2��Һ�м�ϡ�������dz��ɫ����_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
��֪�����ͬ�壬�������ƣ����û�ѧ����ʽ��������ʵ������
��1��CS2��Na2Sˮ��Һһ����ˮ��Һ����ɫ����ɫ________________________��
��2��P2S5����K2Sˮ��Һ��________________��
��3��Na2S2��Һ�м�ϡHCl������ɫ�ij���________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)CS2��Na2Sˮ��Һһ����ˮ��Һ����ɫ����ɫ________________��
(2)P2S5����K2Sˮ��Һ��________________��
(3)Na2S2��Һ�м�ϡHCl����dz��ɫ�ij���________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����ͬ�壬�������ƣ����û�ѧ����ʽ��������ʵ������
(1)CS2��Na2Sˮ��Һһ����ˮ��Һ����ɫ����ɫ________________��
(2)P2S5����K2Sˮ��Һ��________________��
(3)Na2S2��Һ�м�ϡHCl����dz��ɫ�ij���________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com