
| A��Br2/CCl4��Һ | B��ʯ����Һ | C������KMnO4��Һ | D������ |

 ����A��C
����A��C
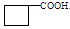 ��
��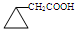 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
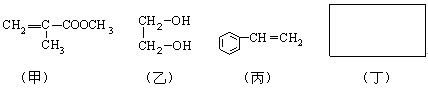
 �����л�����ԭ��R���������Լ�ͨ���ӳɡ�ˮ�⡢���������۷�Ӧ�õ�����R�ǣ� ��
�����л�����ԭ��R���������Լ�ͨ���ӳɡ�ˮ�⡢���������۷�Ӧ�õ�����R�ǣ� ��| A���Ҵ� | B��2-���� | C����ϩ | D��1,3-����ϩ |
�鿴�𰸺ͽ���>>
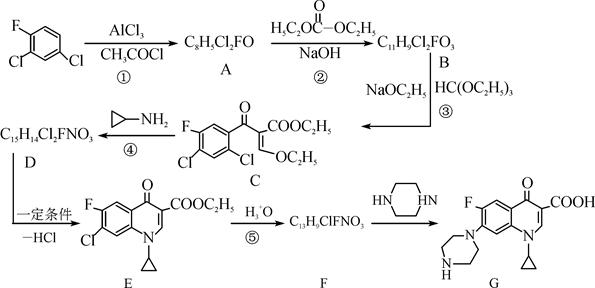
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 RNHCH2 R/+HCl��R��R/����������
RNHCH2 R/+HCl��R��R/����������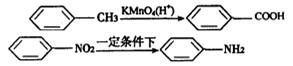
 ��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ���
��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
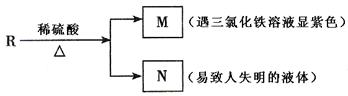
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
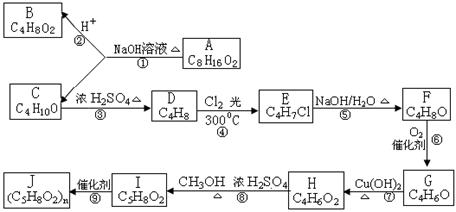
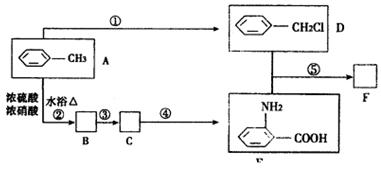
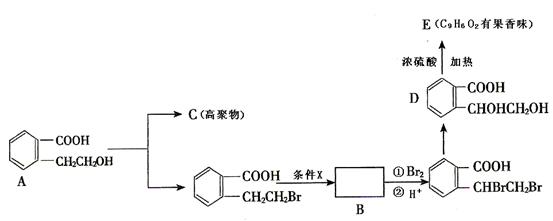
 �� ֪C���ӽṹ��ֻ����һ���⡣FΪ�߷��ӻ�����,�ṹΪ��
�� ֪C���ӽṹ��ֻ����һ���⡣FΪ�߷��ӻ�����,�ṹΪ��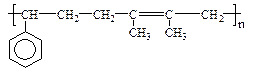 ����������ת����ϵ���£�
����������ת����ϵ���£�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com