

| AЃЎгЩIжаЪ§ОнПЩМЦЫуГіИУЮТЖШЯТЗДгІЕФЦНКтГЃЪ§ЪЧK="20" mol-1?L. |
| BЃЎЂђПЩФмЪЧЭЈЙ§діДѓCЕФХЈЖШЪЕЯжЕФ |
CЃЎШєЂѓжЛЪЧЩ§ИпЮТЖШЃЌ дђгыIБШНЯЃЌПЩвдХаЖЯГіе§ЗДгІвЛЖЈЪЧЗХШШЗДгІ дђгыIБШНЯЃЌПЩвдХаЖЯГіе§ЗДгІвЛЖЈЪЧЗХШШЗДгІ |
| DЃЎЕкЂєзщЪЕбщЪ§ОнЕФЕУГіЃЌЭЈЙ§бЙЫѕШнЦїЕФЬхЛ§ОЭПЩвдЪЕЯж |

| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎвбжЊcЃЈЪЏФЋЃЌsЃЉ=cЃЈН№ИеЪЏЃЌsЃЉЃЛЁїHЃО0ЃЌдђН№ИеЪЏБШЪЏФЋЮШЖЈ |
| BЃЎвбжЊI2ЃЈgЃЉЃЋH2ЃЈgЃЉ=2HIЃЈgЃЉЃЌЁїH1ЃЛI2ЃЈsЃЉЃЋH2ЃЈgЃЉ=2HIЃЈgЃЉЃЌЁїH2ЃЛ дђЁїH1ЃМЁїH2 |
| CЃЎHClКЭNaOHЗДгІЕФжаКЭШШЁїH=Ѓ57ЃЎ3kJ/molЃЌдђH2SO4КЭBaЃЈOHЃЉ2ЗДгІЕФжаКЭШШЁїH=2ЁСЃЈЃ57ЃЎ3ЃЉkJ/mol |
| DЃЎвбжЊH2ЃЈgЃЉЃЋF2ЃЈgЃЉ=2HFЃЈgЃЉЃЛЁїH=Ѓ270kJ/molЃЌдђ2LЗњЛЏЧтЦјЬхЗжНтГЩ1LЧтЦјКЭ1LЗњЦјЮќЪе270kJШШСП |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎaЃМcЃМ0 | BЃЎbЃОdЃО0 | CЃЎ2aЃНbЃМ0 | DЃЎ2cЃНdЃО0 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт
 ЁЃ
ЁЃ
 вЦЯђ ЃЈЬюЁАaЁБЛђЁАbЁБЃЉЕчМЋЃЌ
вЦЯђ ЃЈЬюЁАaЁБЛђЁАbЁБЃЉЕчМЋЃЌ вЦЯђ ЃЈЬюЁА AЁБЛђЁАBЁБЃЉЕчМЋЃЛ
вЦЯђ ЃЈЬюЁА AЁБЛђЁАBЁБЃЉЕчМЋЃЛ ЃЈ3ЃЉввГижаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ ЃЛ
ЃЈ3ЃЉввГижаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ ЃЛ
 (БъзМзДПіЯТ)ЁЃШчКЮМьбщAДІЕФВњЮя ЁЃ
(БъзМзДПіЯТ)ЁЃШчКЮМьбщAДІЕФВњЮя ЁЃ ШлЕуЁЂЗаЕуЪ§ОнШчЯТЃК
ШлЕуЁЂЗаЕуЪ§ОнШчЯТЃК| ЮяжЪ | Al |  | 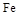 | 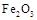 |
| ШлЕу/Ёц | 660 | 2054 | 153 | 14 62 62 |
| ЗаЕу/Ёц | 2467 | 2980 | 2 750 750 | - |
 ЁЃ
ЁЃ ЪдМСжазюЪЪвЫЕФЪдМСЪЧ
ЪдМСжазюЪЪвЫЕФЪдМСЪЧ ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎИУРызгЛЏКЯЮяжа1molбєРызгЙВга15molЕчзг |
| BЃЎдкДЫЗДгІжаO2ЪЧбѕЛЏМСЃЌPtF6ЪЧЛЙдМС |
| CЃЎУПЩњГЩO2PtF61molзЊвЦ2molЕчзг |
| DЃЎИУРызгЛЏКЯЮяжажЛКЌРызгМќ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт

 жаM+жаЫљгаЕчзге§КУГфТњKЁЂLЁЂMШ§ИіЕчзгВуЃЌЫќM+ЕФЗћКХЪЧ ЃЌгыЭЌвЛИіN3ЃЯрСЌЕФM+га ИіЁЃ
жаM+жаЫљгаЕчзге§КУГфТњKЁЂLЁЂMШ§ИіЕчзгВуЃЌЫќM+ЕФЗћКХЪЧ ЃЌгыЭЌвЛИіN3ЃЯрСЌЕФM+га ИіЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
 Саа№Ъіжае§ШЗЕФЪЧЃЈ ЃЉ
Саа№Ъіжае§ШЗЕФЪЧЃЈ ЃЉ| AЃЎMgSO4ОЇЬхжажЛДцдкРызгМќ |
| BЃЎCH4ЁЂCO2ЖМЪЧгЩМЋадМќЙЙГЩЕФ |
| CЃЎМюН№ЪєЮЛгкжмЦкБэжаЂёAжїзхЃЌЦфУмЖШОљаЁгкЫЎ |
| DЃЎДЮТШЫсЕФНсЙЙЪНЃКHЁЊClЁЊO |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎЂйЂкЂм | BЃЎЂйЂлЂо | CЃЎЂкЂмЂо | DЃЎЂлЂмЂн |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎЫсадбѕЛЏЮявЛЖЈЪЧЗЧН№ЪєбѕЛЏЮяЃЌН№ЪєбѕЛЏЮявЛЖЈЪЧМюадбѕЛЏЮя |
| BЃЎЧтЦјжаЧтдЊЫиЕФЛЏКЯМлЮЊ0МлЃЌЫљвдЧтЗжзгжаУЛгаЛЏбЇМќ |
| CЃЎРызгМќжЛДцдкгкРызгЛЏКЯЮяжаЃЌЖјЙВМлМќПЩФмДцдкгкРызгЛЏКЯЮяЛђЙВМлЛЏКЯЮяжа |
| DЃЎЯђAgNO3ШмвКжаЯШЕЮШыЙ§СПЕФNa2SШмвКЃЌдйЕЮШыNaClШмвКЃЌПЩЗЂЩњГСЕэзЊЛЏ |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com