
����16�֣�������������ͼ�ǽṹ��ʽΪCH3CH2CH2OH��CH3CH(OH)CH3�������л��������1H�˴Ź�����ͼ�����ж���һ����CH3CH(OH)CH3��1H��NMR��ͼ����˵�����ɡ���6�֣�
�ڡ�0.2 mol�л����0.4 mol O2���ܱ�������ȼ�պ�IJ���ΪCO2��CO��H2O��g����ȼ�պ����Щ���ᆳ��ŨH2SO4����������10.8 g����ͨ�����ȵ�CuO��ַ�Ӧ������������3.2 g�����������ͨ����ʯ�ұ���ȫ���գ���������17.6 g���������õ������ԭ������H-1��C-12��O-16��Cu-64��
��1���ƶϸ��л���ķ���ʽ����6�֣�
��2����0.2 mol���л�����������Ľ�������ȫ��Ӧ��ų�4.48 L H2����״��������ȷ���л���Ľṹ��ʽ����4�֣�?
��A��3�֣����ɣ��ӽṹ�Ϸ�����CH3CH(OH)CH3�����ֲ�ͬ��ѧ������H����CH3CH2CH2OH�����ֲ�ͬ��H���ʴ�ͼ�Ϸ�����A���������壬��B���ĸ��壬��A��CH3CH(OH)CH3��1H��NMR��ͼ��3�֣���
�ڣ�1��Ũ�������ӵ���������ˮ������������ˮ��10.8g�����ʵ�����0.6mol��COͨ�����ȵ�����ͭ�ֱ���������CO2�������л�����ȫȼ�ղ�����CO2��17.6g�����ʵ�����0.4mol�����ݷ���ʽCO��CuO Cu��CO2��֪��ÿ����1molCO�����������ͼ���16g������CO�����ʵ�����3.2��16��0.2mol�����л���ȼ����������0.2CO2��0.2molCO��0.6molˮ�����Ը���ԭ���غ��֪�л�������ԭ�ӵ����ʵ�����0.4mol+0.2mol+0.6mol-0.4mol��2��0.4mol������л�������ʽΪCH3O����Ϊ2��̼ԭ�������6����ԭ�ӣ����Ի�����ķ���ʽΪC2H6O2.
Cu��CO2��֪��ÿ����1molCO�����������ͼ���16g������CO�����ʵ�����3.2��16��0.2mol�����л���ȼ����������0.2CO2��0.2molCO��0.6molˮ�����Ը���ԭ���غ��֪�л�������ԭ�ӵ����ʵ�����0.4mol+0.2mol+0.6mol-0.4mol��2��0.4mol������л�������ʽΪCH3O����Ϊ2��̼ԭ�������6����ԭ�ӣ����Ի�����ķ���ʽΪC2H6O2.
��2����״����4.48L���������ʵ�����0.2mol����Ϊ2���ǻ�������1���������������л������ǻ��ĸ�����2����������Ҷ������ṹ��ʽΪHOCH2CH2OH��
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����16�֣�
��֪���������������������壩��H2�ڸ�����������ʱҪ�Է�����Ӧ�����������ͽ����⻯���NaH��CaH2�ȣ���������ˮ��Ӧ�����мס�����λͬѧ�ֱ�������Ʊ�CaH2��ʵ�飬װ����ͼ��ʾ���ֱ�����Ţ��ʾ��.
��ش���������
��1��п�����ᷴӦ�����ӷ���ʽΪ ��
��2�����ʵ������ʾ����λͬѧ��ʵ��װ����ƶ���ȱ�ݡ�
װ�â�IJ���֮���� ��
װ�â�IJ���֮���� ��
��3�����������װ����ѡȡ����Ϊ�����IJ��֣��������ҵ�˳����װһ����ȡCaH2�ĺ���װ�ã������A��B��C������ʾ�� ��
��4�������װ�õ������Ժ�Ϊ�˱�֤��Ʒ�Ĵ��Ⱥ�ʵ�鰲ȫ������ ��Ȼ����
�����ܵ�ȼ�ƾ��Ƽ��ȡ�ͬ����Ϊ�˰�ȫ����Ӧ��ʼ����E�ڴ�Ӧ ��
��5�����º�����Ұ����ҵ����CaH2���������ʹ֮��ˮ��Ӧ����H2���䷴Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡΫ���и���3�µ�һ��ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ������
�������A��B��С�⣬��ѡ������һС�Ⲣ����Ӧ�Ĵ�
����������������������AС�����֡�
A�������ʽṹ�����ʡ�
Ԫ��H��C��N��O��F������Ҫ�ķǽ���Ԫ�أ�Fe��Cu��Ӧ�÷dz��㷺�Ľ�����
��1��FeԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽΪ ��
��2��C��HԪ���γɵĻ���������й���16�����ӣ��÷�����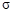 ����
����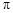 ���ĸ�����Ϊ
���ĸ�����Ϊ
��
��3��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��
��
��4���ڲⶨHF����Է�������ʱ��ʵ����ֵһ���������ֵ������Ҫԭ����
��
��5��C��N��Ԫ���γɵĻ�����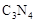 �γɵ�ԭ�Ӿ��壬�ṹ����
�γɵ�ԭ�Ӿ��壬�ṹ����
���ʯ������Ӳ�ȳ������ʯ����ԭ���� ��
��6����ͼΪʯī�����ṹʾ��ͼ���þ����к���Cԭ�ӵĸ���Ϊ ��
B�����л���ѧ������
����Ϣʹ��ѧ�������ᰱ���ӣ��������г��õ���һ�ֽ�����ʹҩ���Ա�Ϊԭ�Ϻϳ�����Ϣ
ʹ�IJ���ת�����£�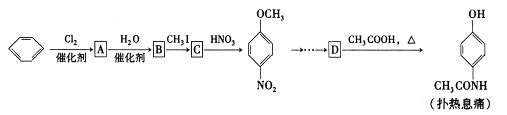
��ش��������⣺
��1��B C�ķ�Ӧ����Ϊ ��D�й����ŵ�����Ϊ ��
C�ķ�Ӧ����Ϊ ��D�й����ŵ�����Ϊ ��
��2��C�Ľṹ��ʽΪ ��
��3��1mol����Ϣʹ������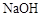 ��Һ��Ӧ������������
��Һ��Ӧ������������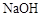 �����ʵ���Ϊ mol��
�����ʵ���Ϊ mol��
��4��ʵ��֤��B�ܹ�����ˮ��Ӧ����д���䷴Ӧ�Ļ�ѧ����ʽ ��
��5������Ϣʹ�ж���ͬ���칹�壬ͬʱ��������Ҫ���ͬ���칹���� �֡�
�ٱ�������������λȡ��������ͬ�ڰ����ᡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡΫ���и���3�µ�һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
�������A��B��С�⣬��ѡ������һС�Ⲣ����Ӧ�Ĵ�
����������������������AС�����֡�
A�������ʽṹ�����ʡ�
Ԫ��H��C��N��O��F������Ҫ�ķǽ���Ԫ�أ�Fe��Cu��Ӧ�÷dz��㷺�Ľ�����
��1��FeԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽΪ ��
��2��C��HԪ���γɵĻ���������й���16�����ӣ��÷����� ����
����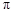 ���ĸ�����Ϊ
���ĸ�����Ϊ
��
��3��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��
��
��4���ڲⶨHF����Է�������ʱ��ʵ����ֵһ���������ֵ������Ҫԭ����
��
��5��C��N��Ԫ���γɵĻ�����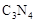 �γɵ�ԭ�Ӿ��壬�ṹ����
�γɵ�ԭ�Ӿ��壬�ṹ����
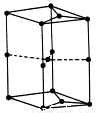
���ʯ������Ӳ�ȳ������ʯ����ԭ���� ��
��6����ͼΪʯī�����ṹʾ��ͼ���þ����к���Cԭ�ӵĸ���Ϊ ��
B�����л���ѧ������
����Ϣʹ��ѧ�������ᰱ���ӣ��������г��õ���һ�ֽ�����ʹҩ���Ա�Ϊԭ�Ϻϳ�����Ϣ
ʹ�IJ���ת�����£�

��ش��������⣺
��1��B C�ķ�Ӧ����Ϊ ��D�й����ŵ�����Ϊ ��
C�ķ�Ӧ����Ϊ ��D�й����ŵ�����Ϊ ��
��2��C�Ľṹ��ʽΪ ��
��3��1mol����Ϣʹ������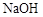 ��Һ��Ӧ������������
��Һ��Ӧ������������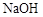 �����ʵ���Ϊ mol��
�����ʵ���Ϊ mol��
��4��ʵ��֤��B�ܹ�����ˮ��Ӧ����д���䷴Ӧ�Ļ�ѧ����ʽ ��
��5������Ϣʹ�ж���ͬ���칹�壬ͬʱ��������Ҫ���ͬ���칹���� �֡�
�ٱ�������������λȡ��������ͬ�ڰ����ᡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ���и߶���ѧ�������ʼ����ۻ�ѧ�Ծ��������棩 ���ͣ������
����16�֣�������������ͼ�ǽṹ��ʽΪCH3CH2CH2OH��CH3CH(OH)CH3�������л��������1H�˴Ź�����ͼ�����ж���һ����CH3CH(OH)CH3��1H��NMR��ͼ����˵�����ɡ���6�֣�

�ڡ�0.2 mol�л����0.4 mol O2���ܱ�������ȼ�պ�IJ���ΪCO2��CO��H2O��g����ȼ�պ����Щ���ᆳ��ŨH2SO4����������10.8 g����ͨ�����ȵ�CuO��ַ�Ӧ������������3.2 g�����������ͨ����ʯ�ұ���ȫ���գ���������17.6 g���������õ������ԭ������H-1��C-12��O-16��Cu-64��
��1���ƶϸ��л���ķ���ʽ����6�֣�
��2����0.2 mol���л�����������Ľ�������ȫ��Ӧ��ų�4.48 L H2����״��������ȷ���л���Ľṹ��ʽ����4�֣�?
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com