���
�⣺��1����Ӧ��-��Ӧ�ۣ��ɵõ���Ӧ�٣����С�H
1=��H
2-��H
3��
��Ӧ��C��s��+CO
2��g��

2CO��g��ƽ�ⳣ��K=
��
��Ӧ��C��s��+H
2O��g��

CO��g��+H
2��g��ƽ�ⳣ��K
1=
��
��Ӧ��CO��g��+H
2O��g��

H
2��g��+CO
2��g�� ƽ�ⳣ��K
2=
��
��Ӧ��-��Ӧ�۵÷�Ӧ�٣�����K=
��
�ʴ�Ϊ����H
1=��H
2-��H
3��
��
��2�����ɱ���֪���¶�Խ�ߣ�ƽ�ⳣ��ԽС����Ӧ���г̶�ԽС��ƽ�����淴Ӧ�ƶ��������¶�ƽ�������ȷ����ƶ���������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
�ڷ�Ӧ��ͬһ�����ڽ��У������ͬ������ʽ�и����ʵĻ�ѧ����������1�������ڼ����о��������ʵ�����ֵ����Ũ����ֵ��800��ʱ��Ӧƽ�ⳣ��Ϊ1��
Qc=
��
A��Qc=
=
��1����Ӧ���淴Ӧ���У���A����
B��Qc=
=
��1����Ӧ������Ӧ���У���B��ȷ��
C����ʼֻ��CO��H
2����Ӧ������Ӧ���У���C��ȷ��
D��Qc=
=1������ƽ��״̬����D����
E��Qc=
=
��1����Ӧ������Ӧ���У���E��ȷ��
��ѡ��BCE��
����CO��Ũ�ȱ仯��Ϊc������ʽ����c��ʾ��ƽ��ʱ����ָ�Ũ�ȣ�
CO��g��+H
2O��g��

H
2��g��+CO
2��g����
��ʼ��mol/L����0.02 0.020 0 0
ת����mol/L����c c c c
ƽ�⣨mol/L����0.02-c 0.02-c c c
500��ʱ����Ӧƽ�ⳣ����K=
=9�����c=0.015��
CO���������ת����Ϊ
��100%=75%��
�ʴ�Ϊ��75%��
����800��ʱ��Ӧƽ�ⳣ��Ϊ1��
CO��g��+H
2O��g��

H
2��g��+CO
2��g����
��ʼ��5��1-x�� 5x 0 0
ת����5��1-x��y 5��1-x��y 5��1-x��y 5��1-x��y
ƽ�⣺5��1-x����1-y�� 5��x-y+xy�� 5��1-x��y 5��1-x��y
����ƽ�ⳣ��K=
| 5(1-x)y��5(1-x)y |
| 5(1-x)(1-y)��5(x-y+xy) |
=1�����y=x��
�ʴ�Ϊ��x��
���ɷ���ʽCO��g��+H
2O��g��=H
2��g��+CO
2��g����֪����1molCO��Ӧ������1molH
2����ʼͨ��10molCO������ƽ��ʱ��CO��H
2�����ʵ�����Ϊ10mol����ƽ��ȼ����Ϊ
=284.2kJ/mol��
����ʮ�ֽ��淨����CO��H
2�����ʵ���֮�ȣ�

��CO��H
2�����ʵ���֮��Ϊ1.8kJ/mol��1.2kJ/mol=3��2��
����n��CO��=10mol��
=6mol��n��H
2��=10mol-6mol=4mol��
��������ʽ�����ƽ��ʱ����ֵ����ʵ�����
CO��g��+H
2O��g��=H
2��g��+CO
2��g����
��ʼ��10mol 10mol 0 0
ת����4mol 4 mol 4mol 4mol
ƽ�⣺6mol 6mol 4mol 4mol
���Գ���ƽ�ⳣ��ΪK=
=
��
�ʴ�Ϊ��
��

 2CO��g��ƽ�ⳣ��K=
2CO��g��ƽ�ⳣ��K= CO��g��+H2��g��ƽ�ⳣ��K1=
CO��g��+H2��g��ƽ�ⳣ��K1= H2��g��+CO2��g�� ƽ�ⳣ��K2=
H2��g��+CO2��g�� ƽ�ⳣ��K2= H2��g��+CO2��g����
H2��g��+CO2��g���� H2��g��+CO2��g����
H2��g��+CO2��g����


 �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
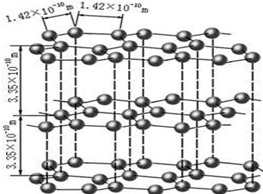
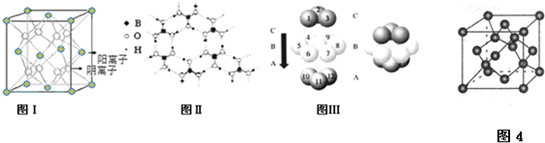
 ����ʯī����14C����CO2��
����ʯī����14C����CO2��
