
(1)������ۻ������Ӽ�˷���________�������������������ۻ������Ӽ�˷���________����������������������Ӽ�˷���________�������������־�����۵��ɸߵ��͵�˳����____________(�ѧʽ)��
(2)�������־��壺��CO2����NaCl����Na����Si����CS2�����ʯ�����ǵ��۵�ӵ͵��ߵ�˳��Ϊ________(�����)��
(3)��H2��(NH4)2SO4��SiC��CO2��HF�У��ɼ��Լ��γɵķǼ��Է�����________���ɷǼ��Լ��γɵķǼ��Է�����________�����γɷ��Ӿ����������________����������ľ���Ļ�ѧʽ��________________________���������Ӿ������____________________������ԭ�Ӿ������____________________���������ʵ��۵��ɸߵ��͵�˳����________________��
(4)A��B��C��DΪ���־��壬�������£�
A����̬ʱ�ܵ��磬����������
B��������CS2��������ˮ
C����̬ʱ�����磬Һ̬ʱ�ܵ��磬������ˮ
D����̬��Һ̬ʱ�������磬�۵�Ϊ3 500��
���ƶ����ǵľ������ͣ�
A��________________��B.________________��
C��________________��D.________________��
(5)��ͬѹǿ�£�����Ԫ�ط�������۵���±���
| ������ | NaF | MgF2 | SiF4 |
| �۵�/�� | 1 266 | 1 534 | 183 |
�Խ����ϱ��з������۵�����ԭ��_____________________________________��
(6)������CO�е��¼��ȣ�������ɫ�ӷ���Һ̬Ni(CO)4���������幹�͡�150 ��ʱ��Ni(CO)4�ֽ�ΪNi��CO��Ni(CO)��________���壬Ni(CO)4����������________(�����)
a��ˮ������b�����Ȼ�̼������c����������d����������Һ
�𰸡�(1)���Ӽ������ۼ������Ӽ䡡SiO2��KClO3��I2
(2)��<��<��<��<��<��
(3)CO2��H2��H2��CO2��HF��HF��(NH4)2SO4��SiC��SiC��(NH4)2SO4��HF��CO2��H2
(4)�������塡���Ӿ��塡���Ӿ��塡ԭ�Ӿ���
(5)NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2���İ뾶��Na���İ뾶С��Mg2����2����λ���������Na���࣬��MgF2���۵��NaF��
(6)���Ӿ��塡bc
������(1)����������Ӿ��壬�ۻ����Ӿ���ʱ��Ҫ�˷����Ӽ���������������������ԭ�Ӿ��壬�ۻ�ԭ�Ӿ���ʱ��Ҫ�˷����ۼ�������������Ϊ���Ӿ��壬�ۻ����Ӿ���ʱ��˷����Ƿ��Ӽ��������������ԭ�Ӿ������ɹ��ۼ��γɵĿռ���״�ṹ�ľ��壬����ԭ�Ӿ�����۵���ߣ���������Ӿ��壻���ڷ��Ӽ��������뻯ѧ����Ƚ�ҪС�ö࣬���Ե���۵���͡�
(2)�Ȱ����־�����ࡣԭ�Ӿ��壺�ܡ��ޣ����Ӿ��壺�ڣ��������壺�ۣ����Ӿ��壺�١��ݡ�����Cԭ�Ӱ뾶С��Siԭ�Ӱ뾶�����Խ��ʯ���۵���ھ���裻CO2��CS2ͬ���ڷ��Ӿ��壬���۵�����Է������������ȣ���CS2�۵����CO2��Na��ͨ��״�����ǹ�̬����CS2��Һ̬��CO2����̬������Na���۵����CS2��CO2��Na��ˮ�м��ۻ���С��˵�������۵��NaCl�͡�
(6)���ݡ��������ܡ�ԭ���ж��ܽ��ԡ�


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͨ�����з����������ܽ�����ʽΪC2H6O���л����ͬ���칹�壬���������ǣ� ��
������� B���˴Ź������� C��ȼ�շ� D�����Ʒ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڵ��ʵľ����У�һ��������(����)
A�����Ӽ�
B�����Ӽ�������
C�����ۼ�
D���������������ɵ��Ӽ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���жԾ��������ж���ȷ����(����)
| ѡ�� | Na2B2O7 | CaF2 | H3BO3 | NH3 |
| A | ԭ�Ӿ��� | �������� | ԭ�Ӿ��� | ���Ӿ��� |
| B | ���Ӿ��� | ���Ӿ��� | ���Ӿ��� | ���Ӿ��� |
| C | ���Ӿ��� | ���Ӿ��� | ���Ӿ��� | ���Ӿ��� |
| D | ���Ӿ��� | ���Ӿ��� | ���Ӿ��� | ���Ӿ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A�����Ӿ����У�ֻ�������Ӽ��������ܴ���������ѧ��
B��������Ԫ��(O��S��Se��Te)���⻯���У�ˮ�ķе����
C��NaHSO4��Na2O2�����е��������Ӹ����Ⱦ�Ϊ1��2
D��������۵㣺���ʯ>̼����>������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��5�ֵ������ӣ����Ƿֱ��� ����
����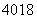 ����
���� ������
������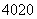 ��2����
��2����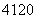 ��(��������Ԫ�ط���δд��)������������Ԫ�ص�������______�֡�
��(��������Ԫ�ط���δд��)������������Ԫ�ص�������______�֡�
(2)1H��2H��3H������������________����Ϊ�����Ǿ�����ͬ______________��ͬһ��ԭ�ӡ�
(3)�� H��
H��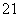 H��
H��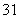 H��
H��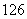 C��
C��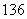 C��
C��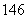 C��
C�� N��
N��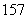 N�У����ء�Ԫ�ص������ֱ�Ϊ______�֡�______�֡�
N�У����ء�Ԫ�ص������ֱ�Ϊ______�֡�______�֡�
(4)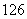 C��
C��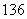 C��
C��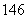 C�Ĺ�ϵΪ__________����Ϊ�����Ǿ���____________________��ͬһԪ�صIJ�ͬԭ�ӡ��仯ѧ���ʼ�����ȫ��ͬ��
C�Ĺ�ϵΪ__________����Ϊ�����Ǿ���____________________��ͬһԪ�صIJ�ͬԭ�ӡ��仯ѧ���ʼ�����ȫ��ͬ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ԫ��ԭ�ӵĺ�����Ӳ���֮��������������֮����ȣ��������ڱ���ǰ10��Ԫ���У�����������ϵ��Ԫ�ع���(����)
A��1�� B��2�� C��3�� D��4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��Ϊ����������Ԫ�أ���˵�����������������ǵ�ԭ������������֮��Ϊ1��4��2��������������ȷ����(����)
A��Xһ���Ǽ����Ԫ��
B��YԪ�����γɻ�����NaHYO3������ˮ��Һ�ʼ���
C��ZԪ���γɵ�����������۵�ϵ�
D��X�ĵ���һ����ͨ�����Z����������Һ�Ʊ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ����ϡ������п��ȡ������ʵ���У����ּ�����������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
(1)����ʵ���з�����Ӧ�Ļ�ѧ����ʽ��_______________________________________
________________________________________________________________________��
(2)����ͭ��Һ���Լӿ������������ʵ�ԭ����______________________________
________________________________________________________________________��
(3)ʵ����������Na2SO4��MgSO4��Ag2SO4��K2SO4��4����Һ����������ʵ����CuSO4��Һ���������õ���________��
(4)Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ��______________________________(������)��
(5)Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ���������һϵ�е�ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
| �� ʵ�� �����Һ������ | A | B | C | D | E | F |
| 4 mol·L��1 H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
������ɴ�ʵ����ƣ����У�V1��__________________��V6��__________��V9��________��
�ڷ�Ӧһ��ʱ���ʵ��A�еĽ�����________ɫ��ʵ��E�еĽ�����________ɫ��
�۸�ͬѧ���ó��Ľ���Ϊ����������CuSO4��Һʱ���������������ʻ�����ߣ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com