
·ÖĪö £Ø1£©Š“³ö“×ĖįµēĄėĘ½ŗā³£Źż”¢“×ĖįøłĄė×ÓĖ®½āĘ½ŗā³£Źż£¬Ė®µÄĄė×Ó»ż³£Źż½ųŠŠ±Č½Ļ£¬“Ó¶ųµĆ³ö¹ŲĻµŹ½£¬½ųŠŠ¼ĘĖć¼“æÉ£»
£Ø2£©½«amol/LµÄHCOOHČÜŅŗÓė$\frac{a}{2}$mol/LµÄNaOHČÜŅŗµČĢå»ż»ģŗĻŗó£¬µĆµ½µÄŹĒ¼×ĖįŗĶ¼×ĖįÄʵĻģŗĻĪļ£¬¾Ż“Ė»Ų“šÅŠ¶Ļ£»
£Ø3£©øł¾ŻČÜŅŗÖŠµÄµēŗÉŹŲŗćŅŌ¼°ĪļĮĻŹŲŗćÖŖŹ¶Ą“¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©Ka=$\frac{c£ØC{H}_{3}CO{O}^{-}£©•c£Ø{H}^{+}£©}{c£ØC{H}_{3}COOH£©}$£¬Kh=$\frac{c£ØC{H}_{3}COOH£©•c£ØO{H}^{-}£©}{c£ØC{H}_{3}CO{O}^{-}£©}$£¬Kw=C£ØH+£©•C£ØOH-£©£¬ĖłŅŌKa•Kh=Kw£¬Kh=$\frac{1{0}^{-14}}{2”Į1{0}^{-4}}$=5”Į10-11£¬
¹Ź“š°øĪŖ£ŗ5”Į10-11£»
£Ø2£©amol/LµÄHCOOHČÜŅŗÓė$\frac{a}{2}$mol/LµÄNaOHČÜŅŗµČĢå»ż»ģŗĻŗó£¬µĆµ½µÄŹĒµČÅØ¶ČµÄ¼×ĖįÄĘŗĶ¼×ĖįµÄ»ģŗĻĪļ£¬ČÜŅŗĻŌŹ¾ĖįŠŌ£¬¼×ĖįµÄµēĄė³Ģ¶Č“óÓŚ¼×ĖįøłĄė×ÓµÄĖ®½ā³Ģ¶Č£¬Ąė×ÓÅØ¶Č“óŠ”Ė³ŠņŹĒ£ŗc£ØHCOO-£©£¾c£ØNa+£©£¾c£ØH+£©£¾c£ØOH-£©£»””
¹Ź“š°øĪŖ£ŗc£ØHCOO-£©£¾c£ØNa+£©£¾c£ØH+£©£¾c£ØOH-£©£»””
£Ø3£©øł¾ŻČÜŅŗĻŌŹ¾ÖŠŠŌ£¬ĖłŅŌc£ØOH-£©=c£ØH+£©£¬øł¾ŻµēŗÉŹŲŗć£¬µĆµ½c£ØHCOO-£©=c£ØNa+£©=$\frac{b}{2}$mol/L£¬øł¾ŻĪļĮĻŹŲŗć£¬ČÜŅŗÖŠc£ØHCOOH£©=$\frac{a}{2}$-c£ØHCOO-£©=$\frac{a-b}{2}$mol/L£¬
¹Ź“š°øĪŖ£ŗ$\frac{a-b}{2}$mol/L£®
µćĘĄ ±¾Ģāæ¼²éĮĖµēĄėĘ½ŗā³£ŹżøÅÄī”¢µēĄėĘ½ŗāÓ°ĻģŅņĖŲŗĶ¼ĘĖćµÄĄķ½āÓ¦ÓĆ”¢ČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”±Č½ĻµČÖŖŹ¶£¬ÕĘĪÕ»ł“”ŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
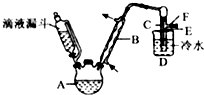 Ā±“śĢžŌŚÉś²śÉś»īÖŠ¾ßÓŠ¹ć·ŗµÄÓ¦ÓĆ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
Ā±“śĢžŌŚÉś²śÉś»īÖŠ¾ßÓŠ¹ć·ŗµÄÓ¦ÓĆ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ| ŅŅ“¼ | äåŅŅĶé | äå | |
| דĢ¬ | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå | Éīŗģ×ŲÉ«ŅŗĢå |
| ĆÜ¶Č£Øg/cm3£© | 0.79 | 1.44 | 3.1 |
| ·Šµć | 78.5 | 38.4 | 59 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
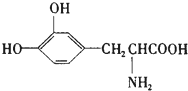 L-¶ą°ĶŹĒŅ»ÖÖÓŠ»śĪļ£¬ĖüæÉÓĆÓŚÅĮ½šÉ×ŪŗĻÖ¢µÄÖĪĮĘ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼£ŗÕāÖÖŅ©ĪļµÄŃŠÖĘŹĒ»łÓŚ»ńµĆ2000ÄźÅµ±“¶ūÉśĄķѧ»ņŅ½Ń§½±”¢2001ÄźÅµ±“¶ū»Æѧ½±µÄŃŠ¾æ³É¹ū£®ĻĀĮŠ¹ŲÓŚL-¶ą°ĶµÄŠšŹöÕżČ·µÄŹĒ£Ø””””£©
L-¶ą°ĶŹĒŅ»ÖÖÓŠ»śĪļ£¬ĖüæÉÓĆÓŚÅĮ½šÉ×ŪŗĻÖ¢µÄÖĪĮĘ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼£ŗÕāÖÖŅ©ĪļµÄŃŠÖĘŹĒ»łÓŚ»ńµĆ2000ÄźÅµ±“¶ūÉśĄķѧ»ņŅ½Ń§½±”¢2001ÄźÅµ±“¶ū»Æѧ½±µÄŃŠ¾æ³É¹ū£®ĻĀĮŠ¹ŲÓŚL-¶ą°ĶµÄŠšŹöÕżČ·µÄŹĒ£Ø””””£©| A£® | Ö»ÄÜÓė¼ī·“Ó¦£¬²»ÄÜÓėĖį·“Ó¦ | |
| B£® | 1moløĆĪļÖŹ×ī¶ąÓė4molNaOH·“Ó¦ | |
| C£® | øĆĪļÖŹ²»ÄÜŹ¹ĖįŠŌKMnO4ĶŹÉ« | |
| D£® | 1moløĆĪļÖŹ×ī¶ąæÉÓė1.5molHBr·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH”ŌCHŗĶ | B£® | ±ūĻ©ŗĶ»·±ūĶé | C£® | 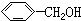 ŗĶ ŗĶ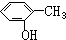 | D£® | ¼×ĆŃŗĶ¼×“¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
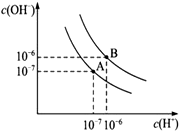 ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ
ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 µÄŗĻ³ÉĀ·ĻߣØĘäĖūĪŽ»śŹŌ¼ĮČĪŃ”£©£®ŅŃÖŖ
µÄŗĻ³ÉĀ·ĻߣØĘäĖūĪŽ»śŹŌ¼ĮČĪŃ”£©£®ŅŃÖŖ £®
£®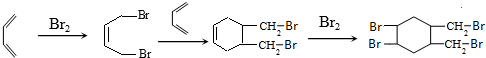 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
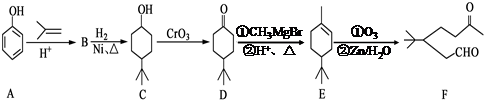
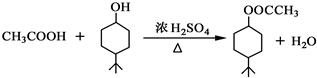 £®
£®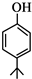 £®Ķ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄBµÄĶ¬·ÖŅģ¹¹Ģå£Ø²»°üĄØB£©¹²ÓŠ11ÖÖ£ŗ
£®Ķ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄBµÄĶ¬·ÖŅģ¹¹Ģå£Ø²»°üĄØB£©¹²ÓŠ11ÖÖ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ŅŃÖŖ25”ꏱ²æ·ÖČõµē½āÖŹµÄµēĄėĘ½ŗā³£ŹżŹż¾ŻČē±ķĖłŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
ŅŃÖŖ25”ꏱ²æ·ÖČõµē½āÖŹµÄµēĄėĘ½ŗā³£ŹżŹż¾ŻČē±ķĖłŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ| »ÆѧŹ½ | CH3COOH | H2CO3 | HClO |
| µēĄėĘ½ŗā³£Źż | Ka=1.8”Į10-5 | Ka1=4.3”Į10-7 Ka2=5.6”Į10-11 | Ka=3.0”Į10-8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com