
ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�����b��d��A2B���⻯���ΪV�η��ӣ�c�ģ�1�����ӱ�e�ģ�1��������8�����ӡ�
�ش��������⣺
(1)Ԫ��aΪ________��cΪ________��
(2)����ЩԪ���γɵ�˫ԭ�ӷ���Ϊ________��
(3)����ЩԪ���γɵ���ԭ�ӷ����У����ӵĿռ�ṹ����ֱ���ε���________����ֱ���ε���________(д2��)��
(4)��ЩԪ�صĵ��ʻ��������γɵ�AB�ͻ������У��侧����������ԭ�Ӿ������________�����Ӿ������________�������������________�����Ӿ������________(ÿ����һ��)��
(5)Ԫ��a��b�γɵ�һ�ֻ�������c��b�γɵ�һ�ֻ�������ķ�Ӧ�����ڷ�������У��÷�Ӧ�Ļ�ѧ����ʽΪ_____________________��
 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������ѧϰ��ѧ����Ҫ���ߣ����л�ѧ�����У��� ȷ����
ȷ����
A��NH4����ˮ�ⷴӦ���ӷ���ʽ��NH4�� + H2O NH3·H2O + H��
NH3·H2O + H��
B����AgCl����Һ�м���KI��Һ�����Ag+ + I- = AgI��
C����ͭʱ�������ĵ缫��ӦʽΪ�� Cu2++2e�� == Cu
D��ij��Ӧ⊿H��0,��÷�Ӧ�����������¾����Է����С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڹ�ҵ�� չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����
չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д����� SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
(1)д��������ҵ���������в���SO2��ʵ����
��________________________________________________________________________��
��________________________________________________________________________��
(2)����SO2��Ⱦ�ɲ��õĴ�ʩ��(д������)��
��________________________________________________________________________��
��________________________________________________________________________��
��___________________ _____________________________________________________��
_____________________________________________________��
(3)ʪʽʯ��ʯ—ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������ Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ—ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�________________________________________________________________________
_____________ ___________________________________________________________��
___________________________________________________________��
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����__________________��
(4)ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4·xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ����ͼ��ʾ�����ݱ��������������Ϊ2.72 g���ٸı䡣��
��ʯ��Ļ�ѧʽ����ͼ����AB�ζ�Ӧ������Ļ�ѧʽ��
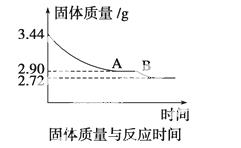
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڵؿ��еĺ����ϸߡ��輰�仯����Ŀ��������Ѿã����ִ��������й㷺Ӧ�á��ش��������⣺
��1��1810����仯ѧ�ұ�������˹�ڼ���ʯӢɰ��ľ̿����ʱ���õ�һ�֡������������֡������������� ��
��2���մɡ�ˮ��Ͳ����dz��õĹ����β��ϡ����У�������ͨ��������Ҫԭ���� ��
��3���ߴ������ִ���Ϣ���� ����������Ȳ�ҵ����Ҫ�Ļ������ϡ���ҵ���ᴿ���ж���·�ߣ�����һ�ֹ�������ʾ��ͼ����Ҫ��Ӧ���£�
����������Ȳ�ҵ����Ҫ�Ļ������ϡ���ҵ���ᴿ���ж���·�ߣ�����һ�ֹ�������ʾ��ͼ����Ҫ��Ӧ���£�
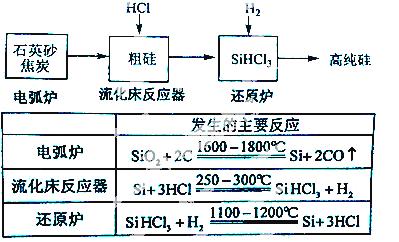
����ʯӢɰ�ͽ�̿�ڵ绡¯�и��¼���Ҳ��������̼���裬�÷�Ӧ�Ļ�ѧ����ʽΪ ��̼�����ֳ� ���侧��ṹ�� ���ơ�
������������Ӧ�IJ����У�SiHCl3��Լռ85%������SiCl4�� SiH2Cl2��SiH3Cl�ȣ��й����ʵķе��������±����ᴿSiHCl3����Ҫ���ղ��������dz����������� ��
SiH2Cl2��SiH3Cl�ȣ��й����ʵķе��������±����ᴿSiHCl3����Ҫ���ղ��������dz����������� ��
| ���� | Si | SiCl4 | SiHCl3 | SiH2Cl2 | SiH3Cl | HCl | Si |
| �е�/�� | 2355 | 57.6 | 31.8 | 8.2 | -30.4 | -84.9 | -111.9 |
��SiHCl3����ˮ�⣬����ȫˮ��IJ���Ϊ ��
��4���ȼҵ��Ϊ�������������ṩ����ԭ�ϣ���Щԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������̿�(��MnO2Լ70%��Al2O3)����п��(��ZnSԼ80%��FeS)��ͬ����MnO2��Zn(�ɵ��ԭ��)��
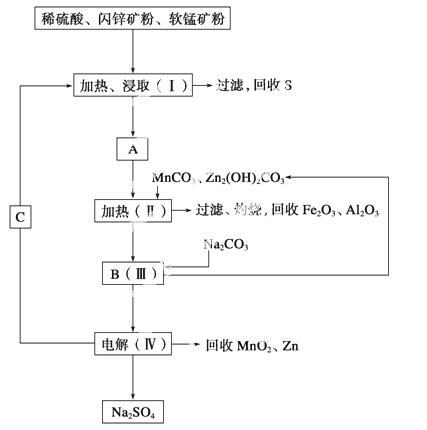
��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
�ڢ��е�ⷽ��ʽΪMnSO4��ZnSO4��2H2O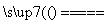 MnO2��Zn��2H2SO4��
MnO2��Zn��2H2SO4��
(1)A�����ڻ�ԭ�������______ ____��
____��
(2)����MnCO3��Zn2(OH)2CO3��������_____________________________________
________________________________________________________________________��
����Ҫ���ȵ�ԭ����____________________________________________________��
C�Ļ�ѧʽ��________________________��
(3)�������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ��________��
(4)��������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ����________��
(5)Ҫ��Na2SO4��Һ�еõ�â��(Na2SO4·10H2O)������еIJ���������Ũ����________�����ˡ�ϴ�ӡ�����ȡ�
(6)������MnO2��Zn�ĽǶȼ��㣬���̿����п��������ȴ�Լ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼Ԫ�صĵ����ж�����ʽ����ͼ������C60��ʯī�ͽ��ʯ�Ľṹͼ��
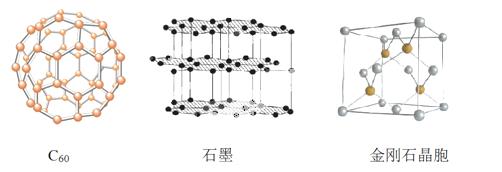
�ش��������⣺
��1�����ʯ��ʯī��C60 .̼���ܵȶ���̼Ԫ�صĵ�����ʽ�����ǻ�Ϊ_____________��
.̼���ܵȶ���̼Ԫ�صĵ�����ʽ�����ǻ�Ϊ_____________��
��2�����ʯ��ʯīϩ��ָ����ʯī����̼ԭ�ӵ��ӻ���ʽ�ֱ�Ϊ____��____��
��3��C60����____���壬ʯī����____���塣
��4��ʯī�����У�����C-C���ļ���Ϊ142 pm�������ʯ��C-C���ļ���Ϊ154 pm����ԭ���ǽ��ʯ��ֻ����C-C���____���ۼ�����ʯī���ڵ�C-C�䲻������____���ۼ�������____����
��5�����ʯ��������____��̼ԭ�ӡ���̼ԭ�Ӱ뾶Ϊr�����ʯ�����ı߳�Ϊa������Ӳ ��Ӵ�ģ�ͣ���r= ______a����ʽ��ʾ̼ԭ���ھ����еĿռ�ռ����____����Ҫ�����������
��Ӵ�ģ�ͣ���r= ______a����ʽ��ʾ̼ԭ���ھ����еĿռ�ռ����____����Ҫ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺��
(1)��̬Niԭ�ӵļ۵���(��Χ����)�Ų�ʽΪ��������;
(2)����������CO�γ������Ni(CO)4,д����CO��Ϊ�ȵ������һ�ַ��Ӻ�һ�����ӵĻ�ѧʽ������������������;
(3)�ܶ�����л�����Ni���¿���H2�����ӳɷ�Ӧ��
���CH2=CH2����HC��CH����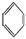 ����HCHO,����̼ԭ�Ӳ�ȡsp2�ӻ��ķ�������������(���������),HCHO���ӵ�����ṹΪ����������;
����HCHO,����̼ԭ�Ӳ�ȡsp2�ӻ��ķ�������������(���������),HCHO���ӵ�����ṹΪ����������;
(4)Ni2+��Fe2+�İ뾶�ֱ�Ϊ69 pm��78 pm,���۵�NiO��������FeO(�<����>��);
(5)����������(La)�γɵĺϽ���һ�����õĴ������,�侧���ṹʾ��ͼ����ͼ��ʾ���úϽ�Ļ�ѧʽΪ��������;
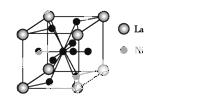
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϱ�������[ KAl(SO4)2·12H2O]�Ļ�ѧ����ʽΪ��
4 KAl(SO4)2·12H2O+3S 2K2SO4+2Al2O3 +9SO2 +48H2O�������ж���ȷ����
2K2SO4+2Al2O3 +9SO2 +48H2O�������ж���ȷ����
A���ڱ��������ķ�Ӧ�У���ԭ���������������ʵ���֮����3��4
B�����õ���K2SO4��Һ�����ԣ�����c(K+)=c(SO42-)
C�����ղ�����SO2������������,����948 t����(M= 474 g/mol)����SO2��������Ϊ96%����������������Ϊ98%������432 t
D����ҵ��ұ��Al2O3�Ƶ�Al����Al��NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2���õ�ط�Ӧ�Ļ�ѧ����ʽ��
Al��3NiO(OH)��NaOH  NaAlO2��3Ni(OH)2
NaAlO2��3Ni(OH)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
12.4 g Na2R��Na��0.4 mol����Na2R��Ħ������Ϊ________��R�����ԭ������Ϊ________����R������Ϊ1.6 g��Na2R�������ʵ���Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com