
�⻯�ƹ����ǵ�ɽ�˶�Ա���õ���Դ�ṩ����ij��ȤС����ѡ������װ���Ʊ��⻯�ơ�
��ش��������⣺
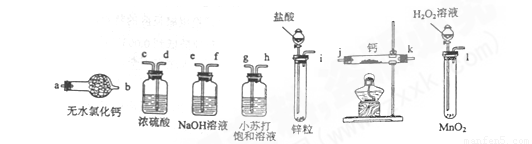
��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ________���������ӿڵ���ĸ��ţ�
��2������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©��������_________���밴��ȷ��˳���������в���ı�ţ���
A�����ȷ�Ӧһ��ʱ�� B���ռ����岢�����䴿��
C���رշ�Һ©������ D��ֹͣ���ȣ������ȴ
��3��ʵ�������ijͬѧȡ�������С�ļ���ˮ�У��۲쵽������ð������Һ�м����̪���Ժ�ɫ����ͬѧ�ݴ��жϣ�����ʵ��ȷ��CaH2���ɡ�
�� д��CaH2��ˮ��Ӧ�Ļ�ѧ����ʽ ___________________;
�ڸ�ͬѧ���жϲ���ȷ��ԭ����_________________;
��4�������ʵ�飬�û�ѧ�������ָ����⻯�ƣ�д��ʵ���Ҫ���輰�۲쵽������______��
��5����ɽ�˶�Ա�����⻯����Ϊ��Դ�ṩ������������ȣ����ŵ���____________��
��10�֣�
��1��i��e��f��d��c��j��k��a ��2�֣�
��2��BADC ��2�֣�
��3����CaH2��2H2O=Ca��OH��2��H2�� ��2�֣�
�ڽ�������ˮ��ӦҲ���������� ��1�֣�
��4��ȡ����������ڼ��������������������Ӧ������Ӧ�������ͨ��װ����ˮ����ͭ�ĸ���ܣ����۲쵽��ɫ��Ϊ��ɫ�������⻯�ƣ������Ǹơ� ��2�֣�
��5���⻯���ǹ��壬Я������ ��1�֣�
����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�⻯�ƹ����ǵ�ɽ�˶�Ա���õ���Դ�ṩ����ij��ȤС����ѡ������װ���Ʊ��⻯�ơ�
��ش��������⣺
��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ________���������ӿڵ���ĸ��ţ�
��2������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©��������_________���밴��ȷ��˳���������в���ı�ţ���
A�����ȷ�Ӧһ��ʱ�� B���ռ����岢�����䴿��
C���رշ�Һ©������ D��ֹͣ���ȣ������ȴ
��3��ʵ�������ijͬѧȡ�������С�ļ���ˮ�У��۲쵽������ð������Һ�м����̪���Ժ�ɫ����ͬѧ�ݴ��жϣ�����ʵ��ȷ��CaH2���ɡ�
�� д��CaH2��ˮ��Ӧ�Ļ�ѧ����ʽ ___________________;
�ڸ�ͬѧ���жϲ���ȷ��ԭ����_________________;
��4�������ʵ�飬�û�ѧ�������ָ����⻯�ƣ�д��ʵ���Ҫ���輰�۲쵽������______��
��5����ɽ�˶�Ա�����⻯����Ϊ��Դ�ṩ������������ȣ����ŵ���____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ����һ�и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
�⻯�ƹ����ǵ�ɽ�˶�Ա���õ���Դ�ṩ����ij��ȤС����ѡ������װ���Ʊ��⻯�ƣ�
��ش��������⣺
��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ-
���������ӿڵ���ĸ��ţ�
��2������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©������ ���밴��ȷ��˳���������в���ı�ţ���
| A�����ȷ�Ӧһ��ʱ�� | B���ռ����岢�����䴿�� |
| C���رշ�Һ©������ | D��ֹͣ���ȣ������ȴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ɽ�и�������ĩͳһ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
�⻯�ƹ����ǵ�ɽ�˶�Ա���õ���Դ�ṩ����ij��ȤС����ѡ������װ���Ʊ��⻯�ơ�

�ش��������⣺
��1�����װ��E�����ԵIJ��������� ��
��2����������װ����ȡ�⻯��ʱ��������������˳��Ϊi��___��___��___�� �� �� ��a���������ӿڵ���ĸ��ţ���
��3������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©��������__________________���밴��ȷ��˳���������в���ı�ţ���
A�����ȷ�Ӧһ��ʱ�� B���ռ����岢�����䴿��
C���رշ�Һ©������ D��ֹͣ���ȣ������ȴ
��4��д��CaH2��ˮ��Ӧ�Ļ�ѧ����ʽ_________________________________________ ����ɽ�˶�Ա�����⻯����Ϊ��Դ�ṩ������������ȣ����ŵ���_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com