
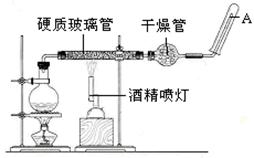
 Fe 3O4 +4H2(��д �����ȡ���1�֣�д�ɡ����¡�����д(g)���۷�) ��H2O��0.4 ��2���� ��3������������ˮ�����ĽӴ��� ���Ƭ���ʯ ��ֹ����
Fe 3O4 +4H2(��д �����ȡ���1�֣�д�ɡ����¡�����д(g)���۷�) ��H2O��0.4 ��2���� ��3������������ˮ�����ĽӴ��� ���Ƭ���ʯ ��ֹ����  Fe 3O4 +4H2��ˮ����Ԫ�صĻ��ϼ۽��ͣ�����ˮ���������� 8.4g����0.15mol������������0.2mol������ת�Ƶ�����0.2mol��2��0.4mol��
Fe 3O4 +4H2��ˮ����Ԫ�صĻ��ϼ۽��ͣ�����ˮ���������� 8.4g����0.15mol������������0.2mol������ת�Ƶ�����0.2mol��2��0.4mol��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ʵ������ | �� �� |
| A | �����ݵ�������Һ�зֱ�μӱ���NaCl��Һ��CuSO4��Һ | ���й������� | �����ʾ��������� |
| B | ������ij��ɫ����ͨ�����ʯ��ˮ | ���ְ�ɫ���� | ������һ����CO2 |
| C | �ֱ��òⶨ������0.1 mol��L��-1 Na2SiO3��Na2CO3��PH | PH:Na2SiO3��Na2CO3 | �ǽ�����Si��C |
| D | ��Ũ�Ⱦ�Ϊ0.1 mol��L��1NaCl��NaI�����Һ�У� �μ�����AgNO3��Һ | ���ֻ�ɫ���� | Ksp(AgCl)��Ksp(AgI) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
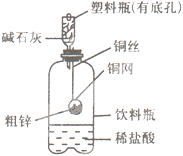
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ����� | ʵ��Ŀ�� |
| A | ���Ҵ��μӵ�����KMnO4��K2Cr2O7��Һ�� | ֤���Ҵ����������� |
| B | �ӵı�����Һ�еμ�ϡ��ˮ | �Ʊ����屽�� |
| C | �����Ѻͱ��ӵĻ��Һ�м�������NaOH������ | ��ȥ�����еı��� |
| D | ����������ˮ�����Һ���Ⱥ����AgNO3��Һ | ����ʳ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������NaOH��Һ����,��ˮ������Һ�м�������,�ټ���AgNO3��Һ����Cl��[ |
| B������ʯ��ˮ��Ӧ�������ͨ����ˮ�У������ˮ��ɫ��֤����Ȳ���巢���˷�Ӧ |
| C��ʵ�������屽ʱ��������Һ���Ϻ�ӵ�����˿�ķ�Ӧ������ |
| D����Ũ��Һ�е�������Ũ��ˮ�ɹ۲쵽��ɫ���屽�ӳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ը��������Һ��ͨ��SO2�������ɫ��ʧ |
| B�����з�̪������������Һ��ͨ��SO2��ɫ��ʧ |
| C��Ʒ����Һͨ��SO2��ɫ��ʧ |
| D����ˮ��ͨ��SO2��ɫ��ʧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ܢ� | B���ڢۢ� | C���ڢۢܢ� | D��ȫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
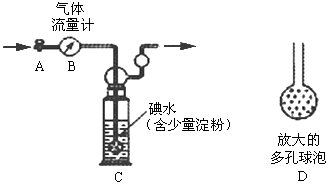
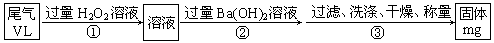
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
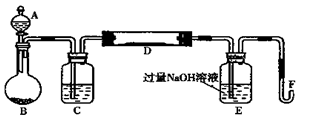
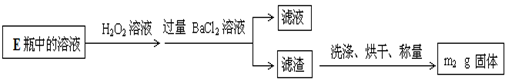
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com