
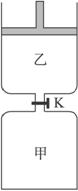
��1������B��ת����Ϊ______________________________��
��2������D������C�����ʵ����ıȽϣ�______________________________�����ȡ���ǰ�ߴ����ߴ�����������______________________________��
��3����K����һ��ʱ�����´ﵽƽ�⣨��ʱ���ҵ����Ϊ���ú�V�Ĵ���ʽ��ʾ����ͨ���е�����������Բ��ƣ�______________________________________________________��
��1��20%
��2�����ߴ� ��ʼʱ��������ѹǿ�Ǽ�������2������Ӧ��ƽ���������ѹǿ�Ǽ�����ѹǿ��2�����࣬�������ʵ�ת���ʽϼ״�ö�
��3��0.29V
������
��1��3A��g��+2B��g��![]() C��g��+2D��g�� ��V
C��g��+2D��g�� ��V
3 2 1 2 2
�� 3 mol 7 mol 0 ?0
ת 2.1 mol? 1.4 mol?0.7 mol?1.4 mol��3+7����(1-0.86) mol=1.4 mol
����B��ת����![]() ��100%=20%
��100%=20%
(3)����������Ϊһ�壬��ԭ�������ǵ�Чƽ�⣬��
![]() ��V(��)=
��V(��)=![]() ��V(��)=
��V(��)=![]() ��0.86 V=1.29 V
��0.86 V=1.29 V
ƽ����ҵ����V(��)=V(��)-V(��)=1.29 V-V=0.29 V��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������2012��������Ĵ���ϻ�ѧ���� ���ͣ�022
����ͼ��ʾ�����رշ���Kʱ������г���1.5 mol��A��3.5 mol��B�������г���3 mol��A��7 mol��B����ʼʱ���ס��������ΪV L������ͬ�¶Ⱥ��д������ڵ������£��������и��Է������з�Ӧ��3A(g)��2B(g)![]() C(g)��2D(g)����H��0�ﵽƽ��(��)ʱ��V(��)��0.86 VL����ش�
C(g)��2D(g)����H��0�ﵽƽ��(��)ʱ��V(��)��0.86 VL����ش�
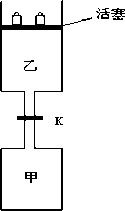
(1)����B��ת����Ϊ________��
(2)����D������C�����ʵ����Ƚϣ�________(���ȡ�����ǰ�ߴ������ߴ�)��
(3)��K����һ��ʱ�����´�ƽ��(��)ʱ���ҵ����Ϊ________(�ú�V�Ĵ���ʽ��ʾ����ͨ��������������Բ���)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com