
ijͬѧ��12.5 mol/L��Ũ��������500 mL��1 mol/L��ϡ���ᣬ�������й�ʵ�飮��ش��������⣺
(1)��Ҫ��ȡŨ����________mL��
(2)���Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ����������⣬�������õ���������________��________�ȣ�
(3)ȡ�����Ƶ�ϡ����100 mL����һ��������п��ַ�Ӧ��пȫ���ܽ�����ɵ������ڱ�״���µ����Ϊ0.896 L����μӷ�Ӧ��п������Ϊ________g���跴Ӧ����Һ�������Ϊ100 mL����Ӧ����Һ��H+�����ʵ���Ũ��Ϊ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(8��)(2011������ģ��)��ȩ������������֯(WHO)ȷ�ϵ��°����ʺ��»�����֮һ���ҹ��涨�����ڼ�ȩ�������ó���0.08mg��m��3��ij�о���ѧϰС�������������KMnO4��Һ�ⶨ��װ�����ڵĿ����м�ȩ�ĺ�����������벢Э�����������ص�ѧϰ����
�ⶨԭ����
����KMnO4Ϊǿ����������������ȩ�Ͳ��ᣬ�䷴Ӧ����ʽ������ʾ��
4MnO4����5HCHO��12H��===4Mn2����5CO2����11H2O
2MnO4����5H2C2O4��6H��===2Mn2����10CO2����8H2O
�ⶨװ�ã�
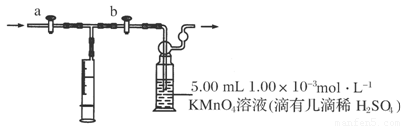
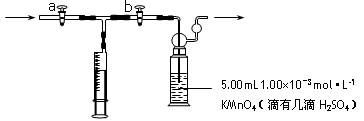
����װ������ͼ��ʾ��
�ⶨ���裺
(1)��______________��ȡ5.00mL 1.00��10��3mol��L��1KMnO4��Һ��ע��ϴ��ƿ�У������뼸��ϡH2SO4����ˮ20mLϡ�ͣ����á�
(2)��1.00��10��3 mol��L��1�IJ������Һ������ʽ�ζ����б��á�
(3)��a���ر�b����ע������ȡ100mL��װ�����ڵĿ������ر�________����________(�a����b��)�����ƶ�ע������������ȫ���������Ը��������Һ�У�ʹ���ַ�Ӧ�����ظ�4�Ρ�
(4)��ϴ��ƿ�е���Һת�Ƶ���ƿ��(����ϴ��Һ)�����ò������Һ���еζ�����¼�ζ������ĵIJ������Һ�������
(5)���ظ�ʵ��2��(ÿ����ȡ�ĸ��������Һ�������Ϊ5.00mL)��3��ʵ�������ĵIJ������Һ�����ƽ��ֵΪ12.38 mL��
�������ۣ�
(1)�������װ�����ڵĿ����м�ȩ��Ũ��Ϊ________mg��m��3������װ�����ڵļ�ȩ________(��ǡ���)���ꣻ
(2)ijͬѧ�ø÷������������м�ȩ�ĺ���ʱ������õ���ֵ��ʵ�ʺ����ͣ����������ܵ�ԭ��(�����������ȡ����Һ���Ƽ��ζ�ʵ�������)����������裺______________��______________(���ٴ�����ֿ�����)��
(3)ʵ�������С���Ա��������Ĺ�����һ����Ϊ��
��ʵ��ԭ�����Լ�
ʵ���пɲ��ò������Һ�ζ����ɶ��ֱ�ӳ�ȡ��װ�����ڵĿ����������͵�ϴ��ƿ�У�ֱ��_________________________________________________________��
��ʵ��װ��Ӧ���ԸĽ�
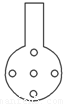
�ɽ���������KMnO4��Һ�еĵ����¶˸ijɾ��ж������(��ͼ��ʾ)�����������ʵ���ȷ�ȣ���������___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��߿���ѧһ�ָ�ϰ����ѧʵ�������ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(8��)(2011������ģ��)��ȩ������������֯(WHO)ȷ�ϵ��°����ʺ��»�����֮һ���ҹ��涨�����ڼ�ȩ�������ó���0.08mg��m��3��ij�о���ѧϰС�������������KMnO4��Һ�ⶨ��װ�����ڵĿ����м�ȩ�ĺ�����������벢Э�����������ص�ѧϰ����
�ⶨԭ����
����KMnO4Ϊǿ����������������ȩ�Ͳ��ᣬ�䷴Ӧ����ʽ������ʾ��
4MnO4����5HCHO��12H��===4Mn2����5CO2����11H2O
2MnO4����5H2C2O4��6H��===2Mn2����10CO2����8H2O
�ⶨװ�ã�
����װ������ͼ��ʾ��
�ⶨ���裺
(1)��______________��ȡ5.00 mL 1.00��10��3mol��L��1KMnO4��Һ��ע��ϴ��ƿ�У������뼸��ϡH2SO4����ˮ20 mLϡ�ͣ����á�
(2)��1.00��10��3 mol��L��1�IJ������Һ������ʽ�ζ����б��á�
(3)��a���ر�b����ע������ȡ100 mL��װ�����ڵĿ������ر�________����________(�a����b��)�����ƶ�ע������������ȫ���������Ը��������Һ�У�ʹ���ַ�Ӧ�����ظ�4�Ρ�
(4)��ϴ��ƿ�е���Һת�Ƶ���ƿ��(����ϴ��Һ)�����ò������Һ���еζ�����¼�ζ������ĵIJ������Һ�������
(5)���ظ�ʵ��2��(ÿ����ȡ�ĸ��������Һ�������Ϊ5.00 mL)��3��ʵ�������ĵIJ������Һ�����ƽ��ֵΪ12.38 mL��
�������ۣ�
(1)�������װ�����ڵĿ����м�ȩ��Ũ��Ϊ________mg��m��3������װ�����ڵļ�ȩ________(��ǡ���)���ꣻ
(2)ijͬѧ�ø÷������������м�ȩ�ĺ���ʱ������õ���ֵ��ʵ�ʺ����ͣ����������ܵ�ԭ��(�����������ȡ����Һ���Ƽ��ζ�ʵ�������)����������裺______________��______________(���ٴ�����ֿ�����)��
(3)ʵ�������С���Ա��������Ĺ�����һ����Ϊ��
��ʵ��ԭ�����Լ�
ʵ���пɲ��ò������Һ�ζ����ɶ��ֱ�ӳ�ȡ��װ�����ڵĿ����������͵�ϴ��ƿ�У�ֱ��_________________________________________________________��
��ʵ��װ��Ӧ���ԸĽ�
�ɽ���������KMnO4��Һ�еĵ����¶˸ijɾ��ж������(��ͼ��ʾ)�����������ʵ���ȷ�ȣ���������___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��߿���ѧһ�ָ�ϰ����ѧʵ�������ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(8��)(2011������ģ��)��ȩ������������֯(WHO)ȷ�ϵ��°����ʺ��»�����֮һ���ҹ��涨�����ڼ�ȩ�������ó���0.08mg��m��3��ij�о���ѧϰС�������������KMnO4��Һ�ⶨ��װ�����ڵĿ����м�ȩ�ĺ�����������벢Э�����������ص�ѧϰ����
�ⶨԭ����
����KMnO4Ϊǿ����������������ȩ�Ͳ��ᣬ�䷴Ӧ����ʽ������ʾ��
4MnO4����5HCHO��12H��===4Mn2����5CO2����11H2O
2MnO4����5H2C2O4��6H��===2Mn2����10CO2����8H2O
�ⶨװ�ã�
����װ������ͼ��ʾ��
�ⶨ���裺
(1)��______________��ȡ5.00 mL 1.00��10��3mol��L��1KMnO4��Һ��ע��ϴ��ƿ�У������뼸��ϡH2SO4����ˮ20 mLϡ�ͣ����á�
(2)��1.00��10��3 mol��L��1�IJ������Һ������ʽ�ζ����б��á�
(3)��a���ر�b����ע������ȡ100 mL��װ�����ڵĿ������ر�________����________(�a����b��)�����ƶ�ע������������ȫ���������Ը��������Һ�У�ʹ���ַ�Ӧ�����ظ�4�Ρ�
(4)��ϴ��ƿ�е���Һת�Ƶ���ƿ��(����ϴ��Һ)�����ò������Һ���еζ�����¼�ζ������ĵIJ������Һ�������
(5)���ظ�ʵ��2��(ÿ����ȡ�ĸ��������Һ�������Ϊ5.00 mL)��3��ʵ�������ĵIJ������Һ�����ƽ��ֵΪ12.38 mL��
�������ۣ�
(1)�������װ�����ڵĿ����м�ȩ��Ũ��Ϊ________mg��m��3������װ�����ڵļ�ȩ________(��ǡ���)���ꣻ
(2)ijͬѧ�ø÷������������м�ȩ�ĺ���ʱ������õ���ֵ��ʵ�ʺ����ͣ����������ܵ�ԭ��(�����������ȡ����Һ���Ƽ��ζ�ʵ�������)����������裺______________��______________(���ٴ�����ֿ�����)��
(3)ʵ�������С���Ա��������Ĺ�����һ����Ϊ��
��ʵ��ԭ�����Լ�
ʵ���пɲ��ò������Һ�ζ����ɶ��ֱ�ӳ�ȡ��װ�����ڵĿ����������͵�ϴ��ƿ�У�ֱ��_________________________________________________________��
��ʵ��װ��Ӧ���ԸĽ�
�ɽ���������KMnO4��Һ�еĵ����¶˸ijɾ��ж������(��ͼ��ʾ)�����������ʵ���ȷ�ȣ���������___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ȩ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ���ҹ��涨�����ڼ�ȩ�������ó���0.08mg��m��3��ij�о���ѧϰС������������KMnO4��Һ�ⶨ�����м�ȩ�ĺ�����������벢Э������������ѧϰ����
�ⶨԭ����KMnO4 ( H��)��ҺΪǿ����������������ȩ�Ͳ��ᡣ
4MnO4�D��5HCHO��H����4Mn2����5CO2����11H2O
2MnO4�D��5H2C2O4��6H����2Mn2����10CO2 ����8H2O
�ⶨװ�ã�����װ������ͼ��ʾ
I���ⶨ���裺
���� ��ȡ5.00mL 1.00��10��3mol��L��1 KMnO4��Һ��ϴ��ƿ�У������뼸��ϡH2SO4����ˮ20mLϡ�ͱ��á�
�ڽ�1.00��10��3 mol��L��1�IJ������Һ������ʽ�ζ����б��á�
�۴� ���ر� ���a����b��������ע������ȡ100mL��װ�ķ������ڿ������ر� ���� ���a����b���������ƶ�ע������������ȫ���������Ը��������Һ�У�ʹ���ַ�Ӧ�����ظ�4�Ρ�
�ܽ�ϴ��ƿ����Һת�Ƶ���ƿ�У�����ϴ��Һ�������ñ�������Һ���еζ�����¼�ζ������ĵIJ�����Һ�������
�����ظ�ʵ��2�Σ�ÿ����ȡ�ĸ��������Һ��Ϊ5.00mL����3��ʵ�������IJ�����Һ�����ƽ��ֵΪ12.38mL��
II���������ۣ��ż���þ����ڿ����м�ȩ��Ũ�� mg��m��3���þ��ҵļ�ȩ �����ǻ���ꡣ
��ijͬѧ�ø÷������������м�ȩ�ĺ���ʱ������õ���ֵ��ʵ�ʺ����ͣ����������ܵ�ԭ������Һ���ơ���������ȡ���ζ�ʵ�����������������裺____________________________________ _�����ٴ��2�ֿ����ԣ���
��ʵ�������С���Ա��������Ĺ�����һ����Ϊ��
��ʵ��װ��Ӧ���ԸĽ���
��ͬѧ���飺�ɽ�����KMnO4��Һ�Ĺ����¶˸ijɾ��ж�����ݣ�����ͼ�������������ʵ���ȷ�ȣ��������� ��
![]()
��ʵ��ԭ�����Լ�ʵ���������ò����Һ�ζ�����ֱ�ӳ�ȡ���ڿ�������ѹ�͵�ϴ��ƿ�У�ֱ��_________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com