
ָ����ʹ�������������Ѿ�ϴ�Ӹɾ�������Ʒʱ�ĵ�һ��������
��ʯ����ֽ���������壩��___________________________________________��
������ƿ��___________________________________________��
����ʽ�ζ��ܣ�___________________________________________��
�ܼ���ƿ���ռ��Ȼ��⣩��___________________________________________��
��2������ʵ��������ʵ���¹ʵ�������ȷ����_________������ţ���
����ϡHNO3��ϴ����������Ӧʵ����Թܣ�
������Ũ�����Ũ����Ļ����ʱ����Ũ�����������������뵽Ũ�����У������Ͻ��裻
���ü�ʽ�ζ�����ȡ20.00 mL 0.1000 mol/L KMnO4��Һ��
����������ƽ��ȡ10.50 g�����NaCl���壻
�ݲ�����Һ��մ��Ƥ���ϣ������þƾ���ϴ��
���ô�������������NaOH��Na2CO3���壻
������ڵ�NaOHϡ��Һ�еμ�FeCl3������Һ�����Ʊ�Fe(OH)3���壻
������Al2(SO4)3��Һʱ������������ϡ���
��ŨH2SO4մ��Ƥ���ϣ�������NaOH��Һ��ϴ��
����������ϡHNO3��Ag������ѧ��Ӧ��ͨ����ϡHNO3��ϴ����������Ӧ���Թܣ�����ȷ����Һ���ʱ��ѭ�ܶȴ��Һ��ӵ��ܶ�С��Һ���У�����ȷ��KMnO4����ǿ�����ԣ����������ʲ����ü�ʽ�ζ�����ȡKMnO4��Һ���۴���������ƽ����С�ֶ�ֵΪ0.1 g�����ܳ���ȷ��0.01 g����Ʒ���ܴ����嵥���и�ʴ�ԣ�����մ��Ƥ���ϣ�Ӧ�þƾ������ܽ��������ȷ������������SiO2,SiO2����NaOH��Na2CO3�ȼ��������ڸ����·�Ӧ��������NaOH��Һ�м���FeCl3������Fe(OH)3�������Ʊ�Fe(OH)3����Ӧ���ˮ�м��뱥��FeCl3��Һ���ߴ���Ϊ����Al2(SO4)3��ˮ�⣬�ɼ�����ϡH2SO4������ȷ��ŨH2SO4մ��Ƥ���ϣ�Ӧ���ò���ȥ�����ô���ˮ��ϴ�������
�𰸣���1����������ˮ��ʪ �ڼ��������ԣ����Ƿ�©ˮ�� �ۼ����Ƿ�©ˮ �ܼ�������� ��2���٢ڢݢ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʯ����ֽ�������� | ����ƿ | ����ƿ�ռ����� |
������ˮʪ�� ������ˮʪ�� |
�������ƿ�Ƿ�©ˮ �������ƿ�Ƿ�©ˮ |
���O��ƿ ���O��ƿ |
| ����NaOH������/g | �Ѹ����� | ���Ѹ��������Ҫ���������� |
4.0 4.0 |
�ձ���������ƽ��ҩ�� | ��������500ml����ƿ����ͷ�ιܡ�Ͳ�������á�Ҳ�ɲ��ã� ��������500ml����ƿ����ͷ�ιܡ�Ͳ�������á�Ҳ�ɲ��ã� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ʯ����ֽ(��������)����?
�ڵζ��ܣ� ��?
�ۼ���ƿ(�ռ�����)�� ��?
��������ƽ ��?
(��)Ŀǰ�����ͼ۾Ӹ߲��¡��Ҵ�������ָ�������м���10%���Ҵ�(�����)���Ҵ����;�������ֵ�ߡ������Ժõ��ص㣬�ƹ�ʹ�ÿɻ�����Դ��ȱ���ٽ����÷�չ�����������ӵ��Ҵ����ܺ�ˮ�������Ӱ�췢����������ת��ʹ���������Ʊ���ˮ�Ҵ��ɲ�ȡ���·�����??
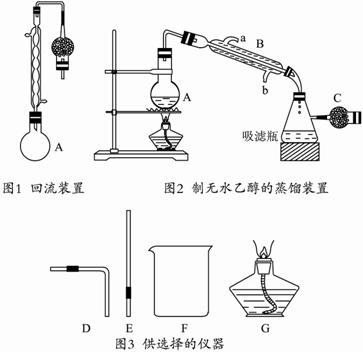
����250 mLԲ����ƿ�м���95%���Ҵ�100 mL�����Ƶ���ʯ��30 g����ˮԡ�м��Ȼ���1��2Сʱ��(��ͼ1��ʾ)?
��ȡ�������ܣ��ij���ͼ2��ʾ��װ�ã��ٽ�A����ˮԡ������?
�۰����������5 mL���Һ������ա�?
���ú�ɵ�����ƿ��Ϊ�����������ܽ�һ֧װ��CaCl2�ĸ����C��ʹ���������ͨ��������Һ�γ���Ϊֹ������99.5%�ľƾ����Իش�?
(1)��֪��101 kPa��25 ��ʱ��?
C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l);��H =-1 367 kJ��mol-1??
1 g�Ҵ���ȫȼ������Һ̬ˮʱ�ų� ������?
(2)ͼ2�еĸ����C�������� ������B�������� ������ˮ�Ǵӿ� (�a����b��)���������ܡ�?
(3)��ˮCaCl2��������ˮ��������ƿA���ܷ�����ˮCaCl2������ʯ�ң� (��д���ܡ������ܡ�)��ԭ���� ��?
(4)�������ò�Ʒ���Ƿ�ˮ�IJ�������������������������������������������������
(5)ijͬѧ��Ҫ����ͼ2װ�õ������ԣ����ɴ�ͼ3��ѡ���ļ�������(����������) ����������������ԵIJ������� ��?
(6)д����������ת��Ϊ�ƾ��Ļ�ѧ����ʽ�� ��?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012������������и߶���ѧ�ڵڶ���ģ���⻯ѧ�Ծ����������� ���ͣ�ʵ����
�������֣�ָ����ʹ�������������Ѿ�ϴ�Ӹɾ�������Ʒʱ�ĵ�һ��������
��1��ʯ����ֽ���������壩�� ��
��2������ƿ�� ��
��3����ʽ�ζ��ܣ� ��
��4������ƿ���ռ��Ȼ��⣩�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������������Ǹ���ѧ������ѧ�ڵڶ����¿���ѧ�Ծ����������� ���ͣ�ʵ����
(9��)(1)ָ����ʹ����������(��ϴ��)����Ʒ�ĵ�һ��������
| ʯ����ֽ�������� | ����ƿ | ����ƿ�ռ����� |
| | | |
| Ӧ����NaOH������/g | �Ѹ����� | ���Ѹ��������Ҫ���������� |
| | �ձ���������ƽ��ҩ�� | |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com