
(1)ŹŌŠ“³öAÖĮFµÄ·Ö×ÓŹ½£ŗA________£»B________£»C________£»D________£»E________£»F________”£
(2)Š“³ö¹żĮæEŗĶNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ________________________”£
Š“³öAŗĶF·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ________________________”£
Š“³öCŗĶB·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ________________________”£
Š“³öDŗĶE·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ________________________”£
(1)NaHSO4 HCl
(2)SO2+![]()
![]() H++
H++![]()
![]() H2O+SO2ӟ
H2O+SO2ӟ
![]() Fe2++H2Sӟ 2H2S+SO2
Fe2++H2S”ü 2H2S+SO2![]() 3S”ż+2H2O
3S”ż+2H2O
½āĪö£ŗÓÉ”°ÄĘŃĪA+NaCl”śB”ü”±ÖŖAŅ»¶ØĪŖĖįŹ½ŃĪ£¬BĪŖHClĘųĢ壬ÓÉ”°C+HCl”śH2S+µĀĢÉ«ČÜŅŗ”±ÖŖCĪŖFeS£¬ÓÉ”°H2S+E”śH2O+µ»ĘÉ«·ŪÄ©”±ÖŖ£ŗµ»ĘÉ«·ŪÄ©ĪŖS£¬EĪŖSO2£¬ÓÉ”°NaOH+SO2(¹żĮæ) ”śF”±ÖŖFĪŖNaHSO3£¬ÓÉA+NaHSO3”śSO2ÖŖAĪŖĒæĖįĖįŹ½ŃĪ£¬Ö»ÓŠNaHSO4”£¹ŹAĪŖNaHSO4”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010”Ŗ2011ѧğø£½ØŹ”°²ĻŖŅ»ÖŠ”¢ŃųÕżÖŠŃ§ø߶žĻĀѧʌʌĩĮŖæ¼»ÆѧŹŌ¾ķ£ØĄķ£© ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©
(1)ĮņĖįļ§ŌŚĒæČČĢõ¼žĻĀ·Ö½ā£¬Éś³É°±”¢¶žŃõ»ÆĮņ”¢µŖĘųŗĶĖ®£¬Ęä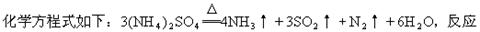
³ÉµÄŃõ»Æ²śĪļÓė»¹Ō²śĪļ·Ö×ÓøöŹżÖ®±ČĪŖ_______________”£
(2)ijÄĘŃĪAµÄČÜŅŗ,ŹµŃ鏱ӊŅŌĻĀŅ»ĻÖĻó:(1)¼ÓČėBa(NO3)2ČÜŅŗŹ±³öĻÖ°×É«³Įµķ,(2)¼ÓČėNa2CO3,ÓŠĘųÅŻ²śÉś,ÓÉ“ĖæÉÅŠ¶Ļ³öøĆÄĘŃĪAĪŖ___________(»ÆѧŹ½),Š“³öÓŠ¹ŲĄė×Ó·½³ĢŹ½_____________________________£¬____________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğøŹĖąŹ”°×ŅųŹŠŗćѧŠ£øßČżµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠÓŠ¹ŲĪļÖŹ·ÖĄą»ņ¹éĄąÕżČ·µÄŅ»×éŹĒ(””””)
¢ŁŅŗ°±”¢ŅŗĀČ”¢øɱł”¢µā»ÆŅų¾łĪŖ»ÆŗĻĪļ
¢ŚĒā·śĖį”¢ŃĪĖį”¢Ė®²£Į§”¢°±Ė®¾łĪŖ»ģŗĻĪļ
¢ŪĆ÷·Æ”¢Š”ĖÕ“ņ”¢“×Ėį”¢“ĪĀČĖį¾łĪŖµē½āÖŹ
¢Üµā¾Ę”¢Å£ÄĢ”¢¶¹½¬”¢ĘÆ·Ū¾«¾łĪŖ½ŗĢå
¢ŻNa2O2”¢Na2CO3”¢NaHCO3”¢Na2SiO3¾łĪŖÄĘŃĪ
A.¢ŁŗĶ¢Ś B.¢ŚŗĶ¢Ū C.¢ŪŗĶ¢Ü D.¢Ś¢Ū¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«Ź”¼ĆÄžŹŠøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠÓŠ¹ŲĪļÖŹ·ÖĄą»ņ¹éĄąÕżČ·µÄŅ»×éŹĒ(””””)
¢ŁŅŗ°±”¢ŅŗĀČ”¢øɱł”¢µā»ÆŅų¾łĪŖ»ÆŗĻĪļ
¢ŚĒā·śĖį”¢ŃĪĖį”¢Ė®²£Į§”¢°±Ė®¾łĪŖ»ģŗĻĪļ
¢ŪĆ÷·Æ”¢Š”ĖÕ“ņ”¢“×Ėį”¢“ĪĀČĖį¾łĪŖµē½āÖŹ
¢Üµā¾Ę”¢Å£ÄĢ”¢¶¹½¬”¢ĘÆ·Ū¾«¾łĪŖ½ŗĢå
¢ŻNa2O2”¢Na2CO3”¢NaHCO3”¢Na2SiO3¾łĪŖÄĘŃĪ
A.¢ŁŗĶ¢Ś B.¢ŚŗĶ¢Ū C.¢ŪŗĶ¢Ü D.¢Ś¢Ū¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijÄĘŃĪAŗĶŹ³ŃĪ¹ĢĢå»ģŗĻĪļ¼ÓČČÉś³ÉŅ»ĘųĢåB£¬½«BČÜÓŚĖ®ÖĘ³É½ĻÅØČÜŅŗŗóŗĶŗŚÉ«¹ĢĢåC·“Ó¦ÖʵĆÓŠ³ō¼¦µ°ĘųĢåµÄĘųĢåDŗĶµĀĢÉ«ČÜŅŗ£¬DĘųĢåŗĶEĘųĢåŌŚ³£ĪĀĻĀ·“Ó¦µĆµ»ĘÉ«·ŪÄ©ŗĶĖ®£¬¹żĮæEĘųĢåĶØČėNaOHČÜŅŗÖŠµĆFČÜŅŗ£¬½«AŗĶFČÜŅŗ»ģŗĻ·“Ó¦ÓÖæɵĆEĘųĢ唣
ŹŌ»Ų“š£ŗ
£Ø1£©ŹŌŠ“³öAÖĮFµÄ»ÆѧŹ½£ŗ””
A______£¬B______£¬C______£¬D_______£¬E_______£¬F_______”£
£Ø2£©Š“³ö¹żĮæEŗĶNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½_________________
Š“³öAŗĶF·“Ó¦µÄĄė×Ó·½³ĢŹ½_____________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com