
������ʵ��д�����з�Ӧ���Ȼ�ѧ��Ӧ����ʽ��
��1����25�桢101kPa�£�1g�״���ȫȼ������CO2��Һ̬ˮʱ����22��68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��2����������N2��O2��ȫ��Ӧ��ÿ����23gNO2��Ҫ����16��95kJ���������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3����NA��ʾ�����ӵ���������C2H2����̬����ȫȼ������CO2��Һ̬ˮ�ķ�Ӧ�У�ÿ��5NA������ת��ʱ���ų�650kJ�����������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ _________________________________________________��
��4����֪��1molH��H����1molN��H����1molN��N���ֱ���Ҫ��������436kJ��391kJ��946kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ ��
��1��CH3OH��l��+1��5O2��g���TCO2��g��+2H2O��l����H=-725��8 kJ?mol-1��
��2��N2��g��+2O2��g��=2NO2��g����H=+67��8kJ?mol-1��
��3��C2H2��g��+O2��g����2CO2��g��+H2O��l����H=-1300kJ?mol-1��
��4��N2��g��+3H2��g�� 2NH3��g����H=-92kJ?mol-1��
2NH3��g����H=-92kJ?mol-1��
���������������1����25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22��68kJ��1mol�״���ȫȼ�����ɶ�����̼��Һ̬ˮ����Ϊ22��68kJ��32=725��8KJ�����Լ״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OH��l��+1��5O2��g���TCO2��g��+2H2O��l����H=-725��8 kJ?mol-1��
��2��������N2��O2��ȫ��Ӧ��ÿ����23��NO2��Ҫ����16��95kJ����������ÿ����92��NO2��Ҫ����67��8kJ���������Ȼ�ѧ����ʽΪN2��g��+2O2��g��=2NO2��g����H=+67��8kJ?mol-1��
��3����3����C2H2����̬����ȫȼ������CO2��Һ̬ˮ�ķ�Ӧ�У�ÿ��5NA������ת��ʱ���ų�650kJ��������������10NA������ת��ʱ���ų�1300kJ��������
���Ȼ�ѧ����ʽΪ��C2H2��g��+2��5O2��g����2CO2��g��+H2O��l����H=-1300kJ?mol-1��
��4���ڷ�ӦN2+3H2?2NH3�У�����3molH-H����1molN��N�������յ�����Ϊ3��436kJ+946kJ=2254kJ������2molNH3�����γ�6molN-H�����ų�������Ϊ6��391kJ=2346kJ�����յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ���ų�������Ϊ2346kJ-2254kJ=92kJ��N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g�� 2NH3��g����H=-92kJ?mol-1��
2NH3��g����H=-92kJ?mol-1��
���㣺�Ȼ�ѧ����ʽ����д


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ĿǰΪֹ���ɻ�ѧ��ת��Ϊ���ܻ������Ȼ������ʹ������Ҫ����Դ��
��1����ѧ��Ӧ�зų������ܣ��ʱ䣬��H���뷴Ӧ����������ڷ�Ӧ�����жϼ����γ��¼����������պͷų������Ĵ�С�йء�
��֪��H2��g����Cl2��g��=2HCl��g�� ��H����185 kJ/mol������1 mol H��H�����յ�����Ϊ436 kJ������1 mol Cl��Cl�����յ�����Ϊ247 kJ�����γ�1 mol H��Cl���ų�������Ϊ ��
��2��ȼ��ȼ�ս��������Ļ�ѧ��ת��Ϊ��������Ҫ�����ܡ���֪��
��CH4��g����2O2��g��=CO2��g����2H2O��l�� ��H����890��3 kJ��mol-1
��C��s,ʯī����O2��g��=CO2��g�� ��H����393��5 kJ��mol��1
��2H2��g����O2��g��=2H2O��l�� ��H����571��6 kJ��mol-1
��״����22��4 L�����ͼ���Ļ�������������������г��ȼ�շ�Ӧ�ų�588��05 kJ��������ԭ��������������������� ���������������Ȼ�ѧ����ʽ������C��s,ʯī����2H2��g��=CH4��g���ķ�Ӧ�Ȧ�HΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��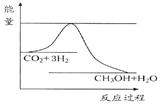
��1�����ڸ÷�Ӧ������˵���У����H 0��(����ڡ�����С�ڡ����ڡ�)�� ���� ����ϸߡ��ϵ͡����¶��������ڸ÷�Ӧ�Է����С�
��2���÷�Ӧƽ�ⳣ��K�ı���ʽΪ ��
��3���¶Ƚ��ͣ�ƽ�ⳣ��K (����������䡱��С��)��
��4����Ϊ�����ݻ���ͬ���ܱ�����,����������г���1 mol CO2(g)��3 molH2(g)���������г���1mol CH3OH(g)��1 mol H2O(g)������ͬ���¶��½��з�Ӧ,�ﵽƽ��ʱ,��������n(CH3OH)���� (����ڡ���С�ڡ����ڡ�)��������n(CH3OH)��
��5����֪��CO(g)+2H2(g) = CH3OH (g) ��H=" -116" kJ?mol-1��CO(g)+1/2O2(g)=CO2(g) ��H="-283" kJ?mol-1��H2 (g)+1/2O2(g)=H2O(g) ��H="-242" kJ?mol-1 ,д��CH3OHȼ������CO2��ˮ�������Ȼ�ѧ����ʽ______________________________________��
��6���Լ״�������Ϊȼ�ϣ�����������ҺΪ�������Һ���ɵ�ء�
�ٸ����ĵ缫��ӦʽΪ ��
������ʯīΪ�缫���������ͭ��Һ��д�������ܷ�Ӧ����ʽ �����Դ�ȼ�ϵ�ص��200 mL 0.8mol/L������ͭ��Һ��������1.6�״�ʱ�������������� gͭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵ����Ϻ����ḻ��һ��Ԫ�أ��䵥�ʼ��������ڹ�ũҵ������������������Ҫ���á�
��1��һ���¶��£���1L�ݻ��㶨���ܱ������г���2 mol N2��8molH2��������Ӧ��10min��ƽ�⣬��ð�����Ũ��Ϊ0��4 mol��L��1����ʱ������ת����Ϊ________��������߰����IJ��ʣ����ݻ�ѧƽ���ƶ�ԭ������������Ľ���______________��д��һ�����ɣ���
��2����ͼ��1mol NO2��g����1mol CO��g����Ӧ����lmol CO2��g����1 mol NO��g�������������仯ʾ��ͼ����д���÷�Ӧ���Ȼ�ѧ����ʽ_____________________��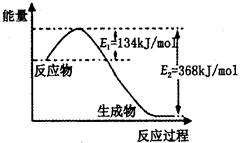
��3�����ݻ��㶨���ܱ������У��������·�Ӧ��N2��g����3H2��g�� 2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���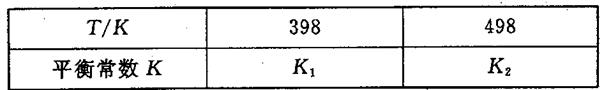
�ٸ÷�Ӧ��ƽ�ⳣ������ʽ��K��_____________��
�����ж�K1__________K2����д��������������������
��NH3��g��ȼ�յķ���ʽΪ��4NH3��g����7O2��g����4NO2��g����6H2O��l������֪��
��2H2��g����O2��g��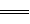 2H2O��l�� ��H����483��6 kJ��mol
2H2O��l�� ��H����483��6 kJ��mol
��N2��g����2O2��g��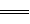 2NO2��g�� ��H����67��8 kJ��mol
2NO2��g�� ��H����67��8 kJ��mol
��N2��g����3H2��g��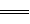 2NH3��g�� ��H����92��0 kJ��mol
2NH3��g�� ��H����92��0 kJ��mol
�����NH3��g����ȼ����________kJ��mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�����Ʊ��״����÷�Ӧ���Ȼ�ѧ����ʽΪ��CO(g)+2H2(g) CH3OH(g) ��H
CH3OH(g) ��H
��֪ijЩ��ѧ���ļ����������±���
| ��ѧ�� | C��C | C��H | H��H | C��O | C��O | H��O |
| ����/kJ��mol��1 | 348 | 413 | 436 | 358 | 1072 | 463 |
| ��Ӧʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 |
| ѹǿ/MPa | 12��6 | 10��8 | 9��5 | 8��7 | 8��4 | 8��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ�������·�����Ӧ��CO(g)��2H2(g) CH3OH(g)����ҵ��������CO����ȼ�ϼ״���
CH3OH(g)����ҵ��������CO����ȼ�ϼ״���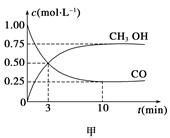
��1����ͼ�Ƿ�ӦʱCO��CH3OH(g)��Ũ����ʱ��仯������ӷ�Ӧ��ʼ��ƽ�⣬��H2Ũ�ȱ仯��ʾƽ����Ӧ����v(CO)��__________________��
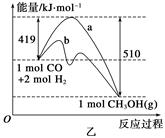
��2����ͼ��ʾ�÷�Ӧ���й����������ı仯������b�µķ�Ӧ����Ϊ ���÷�Ӧ���ʱ���________(���H<0����H>0��)��д����Ӧ���Ȼ�ѧ����ʽ��____________________________________��ѡ�����˵Ĵ���__________(��ܡ����ܡ�)�ı�÷�Ӧ�ķ�Ӧ�ȡ�
��3���÷�Ӧƽ�ⳣ��K�ı���ʽΪ_____________________________________________��
�¶����ߣ�ƽ�ⳣ��K________(����������䡱��С��)��
��4�����������£����д�ʩ����ʹ �������____________��
�������____________��
a�������¶�
b������He��
c���ٳ���1 mol CO��2 mol H2
d��ʹ�ô���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ҵ�������һ������ʳ������ֲ����ά�ӹ��ɵ�ȼ���Ҵ�����ͨ���Ͱ�һ�����������γɵ����������Դ�������ҹ��Ĺ��ұ����Ҵ���������90%����ͨ������10%���Ҵ����Ͷ��ɡ�
��1������ʳ�����ֲ����ά�ɵõ������ǣ�д���������Ƶ��Ҵ��Ļ�ѧ����ʽ: ��
��2���ڳ��³�ѹ�£�1gC2H5OH��ȫȼ������CO2��Һ̬H2Oʱ�ų�29.71 kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
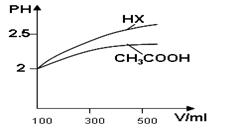
��3����ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺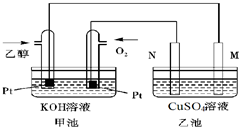
�ټ����Ҵ���Pt�缫�ĵ缫��ӦʽΪ_________________________��
���ڹ��������У��ҳ������缫���ռ�����״����224mL����ʱ���׳����������������������Ϊ mL(��״����)������ʱ�ҳ���Һ���Ϊ200mL�����ҳ�����Һ��pHΪ ��
����Ҫʹ�����ҳص���Һ��ȫ�ָ�����ʼ״̬�������ҳ��м��� (�����)
| A��0.01molCu |
| B��0.01molCuO |
| C��0.01molCu(OH)2 |
| D��0.01molCuCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��Ӧԭ���ڹ�ҵ�����о���ʮ����Ҫ�����塣
��1����ҵ����������NH3��g����CO2��g������������Ӧ�������أ�������Ӧ�������仯ʾ��ͼ���£�
��NH3��g����CO2��g����Ӧ�������ص��Ȼ�ѧ����ʽΪ ��
��2����֪��ӦFe(s) +CO2(g)  FeO(s) +CO(g) ��H ="a" kJ/mol
FeO(s) +CO(g) ��H ="a" kJ/mol
����ڲ�ͬ�¶��£��÷�Ӧ��ƽ�ⳣ��K���¶ȵı仯���£�
�ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��a 0���>������<����=��������500�� 2L�ܱ������н��з�Ӧ��Fe��CO2����ʼ����Ϊ4 mol����5 min��ﵽƽ��ʱCO2��ת����Ϊ ������CO��ƽ������v(CO)Ϊ ��
��700�淴Ӧ�ﵽƽ���Ҫʹ��ƽ�������ƶ���������������ʱ�����Բ�ȡ�Ĵ�ʩ��
������ĸ����
| A����С��Ӧ���ݻ� | B������Fe�����ʵ��� |
| C�������¶ȵ�900�� | D��ʹ�ú��ʵĴ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g)��H���D24��8kJ?mol-1
��3Fe2O3(s)+CO(g)=2Fe3O4(s)+CO2(g)��H���D47��2kJ?mol-1
��Fe3O4(s)+CO(g)=3FeO(s)+CO2(g)��H��+640��5kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��__________________________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com