
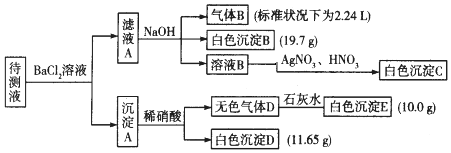
���� ��1����ˮ�к��н϶�ĸ����ӡ�þ���ӣ�����ˮ��Ӳ�Ƚϴ�
��2��ҩ�������������Լ�Ϊ����������ƣ���������ˮ��Ӧ�����������ƣ��������Ƶ������������������þ���ӷ�Ӧ����������þ������̼����������ӷ�Ӧ����̼��Ƴ������ݴ˽��н��
��3���ӷ���¯�г�����������Ҫ���������Ϊ�˷�ֹ�ڽӴ����еĴ����ж���
��� �⣺��1�����ھ�ˮ�к��н϶�Ca2+��Mg2+�����¾�ˮ��Ӳ�Ƚϴ�
�ʴ�Ϊ��D��
��2����������Ӳ�ȵ�Ӳˮ�ɲ���ҩ���������ӽ�������������������ҩ������������ʯ�Һʹ���ȼ���ʯ�ң�������Ӧ��CaO+H2O=Ca2++2OH-���������Ƶ������������������þ���ӷ�Ӧ����������þ������Mg2++2OH-=Mg��OH��2�����ټ���̼���ƽ�������ת����̼��Ƴ�������Ӧ�����ӷ���ʽΪ��CO32-+Ca2+=CaCO3����
�ʴ�Ϊ��ʯ�ң����ʯ�ң�Ҫ��ȥ��þ���ӣ�Ӧ�ȼ�ʯ�ң���Ӵ�����������ĸ����ӿ�ͨ������ʹ֮����������CaO+H2O=Ca2++2OH-��Mg2++2OH-=Mg��OH��2����CO32-+Ca2+=CaCO3����
��3���Ʊ���������У��ӷ���¯�г���������٣��ڴ�����ǰ��Ҫ���������ֹ�ڽӴ����еĴ����ж���
�ʴ�Ϊ���٣���ֹ�Ӵ��ҵĴ����ж���
���� ���⿼����ˮ�ľ����봦�������ӷ���ʽ��д�����Ṥҵ��֪ʶ����Ŀ�ѶȲ�����ȷˮ����������Ϊ���ؼ���ע���������Ṥҵԭ��������������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ�ࣺCaSO4•2H2O���������������ģ�� | |
| B�� | �ؾ�ʯ��BaCO3��������ɫ���ϡ�ҽ���ϡ����͡� | |
| C�� | �̷���FeSO4•7H2O����������ˮ������ֹȱ����ƶѪ��ҩ�� | |
| D�� | ������CuSO4•5H2O������������ũҩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=1����Һ�У�K+ CH3COO- SO42- Na+ | |
| B�� | ����0.1mol/LCa2+����Һ�У�Na+SO42- K+ Cl- | |
| C�� | ����0.1mol/LMg2+����Һ�У�Na+SO42- K+ HCO3- | |
| D�� | �����̪�Ժ�ɫ����Һ��K+ HCO3-Ba2+Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������ | K+��NH4+��Fe3+��Ba2+ |
| ������ | Cl-��CO32-��HCO3-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Al2O3--��Al��OH��3 | B�� | Cu--��CuCl2 | C�� | Fe--��FeCl2 | D�� | Na--��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʯī���缫���AlC13��Һ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$Cl2��+H2��+2OH- | |
| B�� | ���Ը��������Һ��H2O2��Һ��ϣ�2MnO4-+3H2O2+6H+�T2Mn2++4 O2��+6H2O | |
| C�� | ��NaHSO4��Һ�еμ�Ba��OH��2��Һ�����ٲ���������Ba2++OH-+H++SO42-�TBaSO4��+H2O | |
| D�� | ��Fe��NO3��2��NaBr�����Һ�еμ�ϡ���6Br -+2NO3-+8H+�T3Br2+2NO��+4H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com