
������ģ��ʯ����ӹ����ϳ�CH2=CHCOOCH2CH3(��ϩ������) �����ʵĹ��̣�
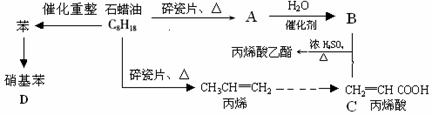
��ش��������⣺
��1��A�ṹ��ʽΪ�������������� ��1�֣�
��2��CH2=CHCOOCH2CH3�ĺ��������� �� �������ƣ���1�֣���
��3��д�����з�Ӧ����ʽ����2�֣���
��B + C��CH2=CHCOOCH2CH3�� �� ��
�ڱ�ϩ���۱�ϩ�� �� ��
�۱����������� �� ��
��4��Ŀǰ�Ʊ��ƾ��ķ��������֣�
![]() ����һ��
����һ��
![]() ��������
��������
�ٶԷ���һ���漰�ķ�Ӧ���ͷֱ�Ϊ���� �� �� ����1�֣����� �� �� ����1�֣�
�ڶ��ڷ�����, M�Ľṹ��ʽΪ�� �� ��1�֣�
������˵����ȷ����_____��_______(�����) ��1�֣�
A������ɫ��ѧ�ĽǶȿ�����һ��ԭ�������ʣ�������
B����ԭ�ϵĿ���������˵�����������
C�������������Ҵ����صĻ�ѧ��������̫����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| NaOH |
| �� |
| ����ø |
| �ƻ�ø |
�鿴�𰸺ͽ���>>
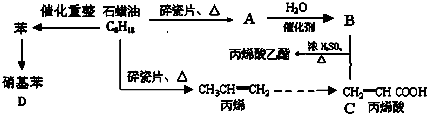
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ɹŰ���һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��14�֣�������ģ��ʯ����ӹ����ϳ�CH2=CHCOOCH2CH3(��ϩ������)�����ʵĹ��̣�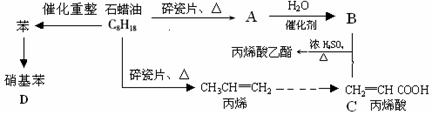
��ش��������⣺
��1��A�Ľṹ��ʽΪ��
��2��CH2=CHCOOCH2CH3�ĺ��������� �������ƣ�
��3��д�����л�ѧ��Ӧ����ʽ
��B+C��CH2=CHCOOCH2CH3��
�ڱ�ϩ���۱�ϩ��
�۱�����������μ��ȣ��� �����ŵ��� ��
��4��Ŀǰ�Ʊ��ƾ��ķ��������֣�
����һ: 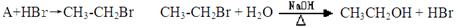
��������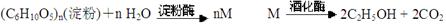
�ٶԷ���һ���漰���л���Ӧ���ͷֱ�Ϊ�� ��
�ڶ��ڷ�����, M�ķ���ʽΪ��
������˵����ȷ����____________(�����) ��1�֣�
A������ɫ��ѧ�ĽǶȿ�����һ��ԭ�������ʣ�������
B����ԭ�ϵĿ���������˵�����������
C�������������Ҵ����صĻ�ѧ��������̫����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����������ƽ���к�ѧУ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��12�֣�������ģ��ʯ����ӹ����ϳ�CH2=CHCOOCH2CH3(��ϩ������) �����ʵĹ��̣�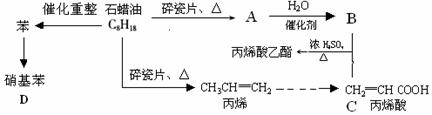
��ش��������⣺
��1��A�ṹ��ʽΪ������������ ��
��2��CH2=CHCOOCH2CH3�ĺ��������� �������ƣ���
��3��д�����з�Ӧ����ʽ��
B+C��CH2=CHCOOCH2CH3�� ��
��ϩ���۱�ϩ�� ��
������������ ��
��4��Ŀǰ�Ʊ��ƾ��ķ��������֣�
����һ��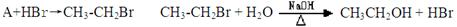
��������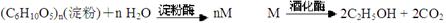
�ٶԷ���һ���漰�ķ�Ӧ���ͷֱ�Ϊ���� �� ���� ����
�ڶ��ڷ�����, M�Ľṹ��ʽΪ��
������˵����ȷ����____________(�����)
A������ɫ��ѧ�ĽǶȿ�����һ��ԭ��������100%
B����ԭ�ϵĿ���������˵�����������
C�������������Ҵ����صĻ�ѧ��������̫����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ɹŰ��и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��14�֣�������ģ��ʯ����ӹ����ϳ�CH2=CHCOOCH2CH3(��ϩ������)�����ʵĹ��̣�
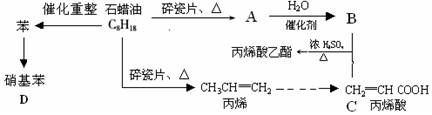
��ش��������⣺
��1��A�Ľṹ��ʽΪ��
��2��CH2=CHCOOCH2CH3�ĺ��������� �������ƣ�
��3��д�����л�ѧ��Ӧ����ʽ
��B+C��CH2=CHCOOCH2CH3��
�ڱ�ϩ���۱�ϩ��
�۱�����������μ��ȣ��� �����ŵ��� ��
��4��Ŀǰ�Ʊ��ƾ��ķ��������֣�
����һ: 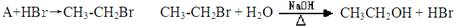
��������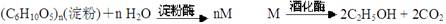
�ٶԷ���һ���漰���л���Ӧ���ͷֱ�Ϊ�� ��
�ڶ��ڷ�����, M�ķ���ʽΪ��
������˵����ȷ����____________(�����) ��1�֣�
A������ɫ��ѧ�ĽǶȿ�����һ��ԭ�������ʣ�������
B����ԭ�ϵĿ���������˵�����������
C�������������Ҵ����صĻ�ѧ��������̫����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���������к�ѧУ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣�������ģ��ʯ����ӹ����ϳ�CH2=CHCOOCH2CH3(��ϩ������) �����ʵĹ��̣�
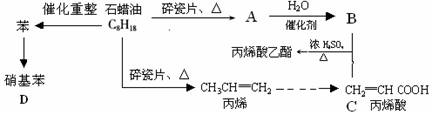
��ش��������⣺
��1��A�ṹ��ʽΪ������������ ��
��2��CH2=CHCOOCH2CH3�ĺ��������� �������ƣ���
��3��д�����з�Ӧ����ʽ��
B+C��CH2=CHCOOCH2CH3�� ��
��ϩ���۱�ϩ�� ��
������������ ��
��4��Ŀǰ�Ʊ��ƾ��ķ��������֣�
����һ��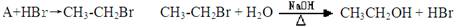
��������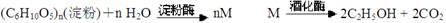
�ٶԷ���һ���漰�ķ�Ӧ���ͷֱ�Ϊ���� �� ���� �� ��
�ڶ��ڷ�����, M�Ľṹ��ʽΪ��
������˵����ȷ����____________(�����)
A������ɫ��ѧ�ĽǶȿ�����һ��ԭ��������100%
B����ԭ�ϵĿ���������˵�����������
C�������������Ҵ����صĻ�ѧ��������̫����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com