
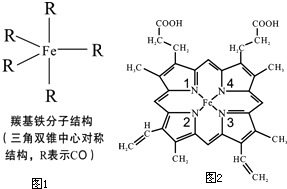
 Fe��C��N��O��H������ɶ������ʣ��ش��������⣺
Fe��C��N��O��H������ɶ������ʣ��ش��������⣺���� ��1������Fe����Χ�����Ų�������
��2���ٸ���CO�ĽṹʽC��O������
�ڵ縺�������������������������ṩ�¶Ե����γ���λ����
����ͼ1��֪�ʻ�������Ϊ����˫���ĶԳƽṹ��������������ص����ڷǼ��Է��ӣ�
��3�����ݷ����еŶԵ��Ӳ����ų�����ǿ�ڳɶԵ�����������
��4���ٷ��Ӽ���ڷ��»����������к����Ȼ����γ������
�ڸ���Nԭ�ӵĹ��ۼ���Ŀ������
��� �⣺��1��FeΪ26��Ԫ�أ�Fe����Χ�����Ų�3d64s2����3d�������4��δ�ɶԵ��ӣ��ʴ�Ϊ��4��
��2����CO�ĽṹʽC��O�������к���1���Ҽ���2���м�����CO�����ЦҼ���м���Ŀ֮��Ϊ1��2���ʴ�Ϊ��1��2��
�ڵ縺�������������������������ṩ�¶Ե����γ���λ������Fe��CO��5����Fe�γ���λ������̼ԭ�ӣ��ʴ�Ϊ��̼��
����ͼ1��֪�ʻ�������Ϊ����˫���ĶԳƽṹ��������������ص����ڷǼ��Է��ӣ�
A��SO2 ����V�η��ӣ�����������IJ��ص����ڼ��Է��ӣ�
B��CS2 ����ֱ���η��ӣ�������������ص����ڷǼ��Է��ӣ�
C��BF3 ����ƽ�������η��ӣ�������������ص����ڷǼ��Է��ӣ�
D��PCl3���������η��ӣ�����������IJ��ص����ڼ��Է��ӣ�
�������ʻ������ӵļ������Ƶķ�����B��C��
�ʴ�Ϊ��B��C��
��3��ˮ��������ԭ�Ӵ��ڹ¶Ե��Ӻͳɼ����Ӷԣ���������в����ڹ¶Ե��ӣ��¶Ե��Ӳ����ų�����ǿ�ڳɶԵ��ӣ�ʹˮ������O-H���ļ��Ǽ�С������CH4�����еļ��Ǵ���H2O���ӵļ��ǣ�
�ʴ�Ϊ������ˮ��������ԭ�Ӵ��ڹ¶Ե��Ӻͳɼ����Ӷԣ���������в����ڹ¶Ե��ӣ��¶Ե��Ӳ����ų�����ǿ�ڳɶԵ��ӣ�
��4����Ѫ���ط��Ӽ���ڷ��»����������к����Ȼ����γ����������Ѫ���ط��Ӽ���ڵ��������������»����������
�ʴ�Ϊ�����»����������
��2��4Nԭ��ֻ�γ�2�����ۼ���������Fe�γ�һ��Ĺ��ۼ���ʹNԭ���γ�8�����ȶ��ṹ��1��3N�γ�3�����ۼ����Ѿ��ﵽ8�����ȶ��ṹ���������γɹ��õ��Ӷԣ����Ǻ��йµ��Ӷԣ����γ���λ����
�ʴ�Ϊ��1��3��
���� ������Ҫ�����˻�̬ԭ�ӵĺ�������Ų������ۼ������͡����ӵļ��ԡ����ǡ��������λ����֪ʶ���Ѷ��еȣ������ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������ע�������λ���γɵ�ԭ���Լ����Ӽ��Ե��жϷ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ӧ��������Ӧ�Լ�����ˮ�������ȡ����Ӧ���� | |
| B�� | �Ҵ��������������ϩ����������ķ�Ӧ������ͬ | |
| C�� | ��������������Һ��ȥ���������л��е�������Ҵ� | |
| D�� | ���顢��������������������ͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH2=CH2+HCl$��_{��}^{����}$CH3CH2Cl | |
| B�� | CH4+Cl2$\stackrel{����}{��}$CH3Cl+HCl | |
| C�� | 2CH3CHO+O2$��_{��}^{����}$2CH3COOH | |
| D�� | CH3CH2Br+NaOH$\stackrel{�Ҵ�}{��}$CH2=CH2��+NaBr+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����18s | B�� | ����12s | C�� | ����18s | D�� | ��18s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �ں����Ƽҵ�У����Ȼ�����Һ����ͨ������̼����ͨ���� | |
| B�� | �����Ṥҵ���ϳɰ���ҵ�����Ṥҵ�У��Բ���ѭ���������ԭ�������� | |
| C�� | ���ȼҵ������һ�����������缫 | |
| D�� | �ϳɰ���ҵ����Ȼ�������ķ����У���ˮ�������·�Ӧ�����ȷֽⷨ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.01mol•L-1H2S��Һ��c��H+����c��HS-����c��S2-����c��H2S����c��OH-�� | |
| B�� | 0.1 mol•L-1NaHSO3��Һ��c��Na+��+c��H+����c��HSO3-��+c��SO32-��+c��OH-�� | |
| C�� | �����ʵ�����NH4Cl��NaCl�Ļ����Һ��c��NH4+��+c��NH3•H2O��+c��Na+��=2c��Cl-�� | |
| D�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=10-10��Na2CO3��Һ��c��HCO3-��+c��H2CO3��=c��10-2-10-12�� mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
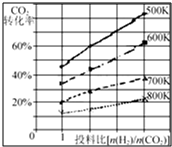 ��һ�������£���ȼú�����е�CO2ת��Ϊ�����ѵķ�ӦΪ��
��һ�������£���ȼú�����е�CO2ת��Ϊ�����ѵķ�ӦΪ��| ʱ�䣨min�� | 5 | 10 | 15 | 20 | 25 | 30 |
| ѹǿ�ȣ�P��/Pǰ�� | 0.98 | 0.90 | 0.80 | 0.70 | 0.70 | 0.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Ca��NO3��2 | B�� | NaCl | C�� | H2O | D�� | KOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com