
�ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�к��д���Mg2+��Al3+��Fe3+��Ag+��Ba2+�����ӡ��Է����ش��������⣺
��1���÷�Һ�п��ܴ������ڵ�һ���������� ������ţ���
A��SO![]() B��NO
B��NO![]() C��Cl
C��Cl![]() D��CO
D��CO![]()
��2��Ϊ����Һ����Ԫ�صĺ����轫��ӷ�ˮ��Ʒ�з��������
���������ˮ�м����������Լ� ����Ԫ�ط����仯�����ӷ���ʽ�ǣ� ��
�ڹ��ˣ�����Һ��ͨ������CO2���������ij������ˡ�ϴ�Ӹ��������
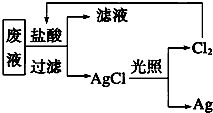
��3����Ҫ���ս��������������һ��ʵ�鷽��������������������д���й���Ҫ�����ӷ�Ӧ����ʽ����
��
��4����ͼΪij�¶��£�Fe(OH)3(s)��Mg(OH)2(s)�ֱ�����Һ�дﵽ�����ܽ�ƽ��ı���Һ��pH��
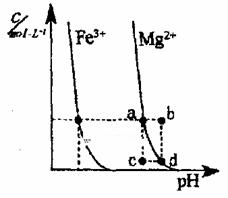
����������Ũ�ȱ仯�������ͼ������
���¶��£��ܶȻ������Ĺ�ϵΪ��
Ksp[Fe(OH)3]____ Ksp[Mg(OH)2] ���������������������������
����������ɵ�Mg(OH)2��Һ�е���������Fe3+����ɫ������ȫ��ת��Ϊ���ɫ������ԭ���ǣ�
_________
_____________________________________ ��
��1��B ��1�֣�
��2��NaOH��1�֣��� Al3+ + 4OH![]() = [Al(OH)4]�����ֲ�дҲ�ɣ�1�֣�
= [Al(OH)4]�����ֲ�дҲ�ɣ�1�֣�
��3�����Һ�мӹ��������ۣ���ֽ������˵�������1�֣������������м���������ᣬϴ�������õ�����1�֣���2Fe3����Fe=3Fe2����Fe��2Ag��=2Ag��Fe2����Fe��2H��=Fe2����H2��
������Ƴɼ����������ᣬ���ˣ��ټ��Ȼ���շֽ��Ȼ���Ҳ�ɣ�Ag++Cl-=AgCl 2AgCl��2Ag+Cl2��ɽ����ѧ����
--------�÷���ʽ��ʾ��һ����1�֣�����Ϊֹ
��4���� ��1�֣�
������Ksp[Fe(OH)3]<Ksp[Mg(OH)2]������Һ�д����ܽ�ƽ�⣺Mg(OH)2==Mg2++2OH-��1�֣���
������Fe3+����OH-���ɸ����ܽ��Fe(OH)3��ʹƽ����������ƶ���1�֣������Mg(OH)2ȫ���ܽ�ת��Ϊ����ɫ��Fe(OH)3��


 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺
����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��2 1.1ˮ�ľ�������ˮ������ϰ���������棩 ���ͣ������
����Ժǿ������ˮ�����֡���������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ��������������⣬ij������Һ�к��д�����Mg2����Al3����Cu2����Ag�����Է����ش��������⡣
(1)�÷�Һ�п��ܴ������ڵ�һ����������________(����ĸ����)��
A��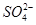 B��
B��
C��Cl�� D��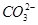
(2)����Һ����Ԫ�صĺ������轫��ӷ�Һ��Ʒ�з�����������õ��Լ�������________��Һ����Ԫ�ط����仯�����ӷ���ʽ��____________________________________________��
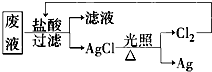
(3)Ϊ�˻��շ�Һ�еĽ�������ijͬѧ��������·�����
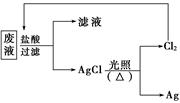
�����÷������Ag 108 g��Ϊ��֤����Ⱦ������Cl2��ѭ�����ã�������Ӧ�ṩ��״���µ�H2�����Ϊ____________��(Ag�����ԭ������Ϊ108)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧ���������� ��ѧ��ɳ�����չ��ϰ���������棩 ���ͣ������
(8��)(1)�ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ����ԭ�����ij������Һ�к��д���Mg2����Al3����Ag����Fe3����Ba2�����Է����ش��������⣺
�ٸ÷�Һ�п��ܴ������ڵ�һ����������____(�����)��
A��SO����B��NO����C��Cl������D��CO
��Ϊ�˳��������Դ�ͱ�����������Ҫ���շ�Һ�еĽ��������������һ������ʵ�鷽������д���й����ӷ�Ӧ����ʽ�� ____________________________________________
________________________________________________________________________��
(2)���ܼ������й����������еĹؼ��ʡ��������糧Ϊ�˼��ٶ���������ŷţ�������ú̿������������������ķ����ж��֣�������һ�ֳ��õ�������
������������ú̿�е�������FeS2���ڵģ���ˮ�Ϳ������ڵ������£�������������������ã�
��2FeS2��7O2��2H2O  4H����2Fe2����4SO42����
4H����2Fe2����4SO42����
��4Fe2����O2��4H�� 4Fe3����2H2O��
4Fe3����2H2O��
��FeS2��2Fe3�� 3Fe2����2S��
3Fe2����2S��
��2S��3O2��2H2O 4H����2SO42����
4H����2SO42����
����������ķ�Ӧ�У�FeS2���ձ�________������ת��Ϊ________(�ѧʽ)����ȥ����Ӧ����������0.2 mol S����ת�Ƶĵ���Ϊ________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com