
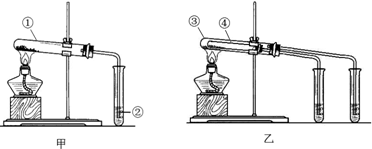
���� NaHCO3���ȶ��������ֽ⣬����ʱ������2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O�����ó���ʯ��ˮ����CO2���ж��Ƿ�ֽ⣬��Na2CO3���ȶ������Ȳ��ֽ⣬��ʵ�����У�Ӧ��NaHCO3װ����װС�Թ��У�С�Թܼ����¶Ƚϵͣ����ֽܷ⣬��֤��NaHCO3���ȶ��������ᷴӦʱ����Ӧ�����ӷ���ʽΪCO32-+H+=HCO3-��HCO3-+H+=CO2��+H2O����NaHCO3���Թܷ�Ӧ�����ң��Դ˽����⣮
��� �⣺�� ��NaHCO3���ȶ��������ֽ⣬����ʱ������2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O�����ó���ʯ��ˮ����CO2���ж��Ƿ�ֽ⣬��������������Ʋ��ܼ��������̼���������ԣ��ʴ�Ϊ��C��
��С�Թܼ����¶Ƚϵͣ����ֽܷ⣬��֤��NaHCO3���ȶ�����Na2CO3�����¶Ƚϸߣ�Ӧ�ù۲쵽��ʵ������������С�Թܵij���ʯ��ˮ����Ƕ����Ӵ��Թܵij���ʯ��ˮ������ǣ���֤��Na2CO3���ȶ���
�ʴ�Ϊ��NaHCO3������С�Թܵij���ʯ��ˮ����Ƕ����Ӵ��Թܵij���ʯ��ˮ������ǣ�ǿ��
�������ᷴӦʱ����Ӧ�����ӷ���ʽΪCO32-+H+=HCO3-��HCO3-+H+=CO2��+H2O����NaHCO3���Թܷ�Ӧ�����ң�
�ʴ�Ϊ��NaHCO3��Һ��
���� ���⿼��̼������̼�����Ƶ����ʱȽϣ��Ǹ߿��еij������ͣ������е��Ѷ�����Ŀ��飮���������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
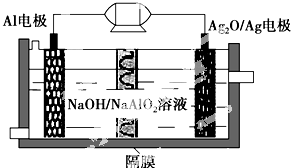 Al-Ag2O�����һ�ֿ�����ˮ�¶�����������Դ����ԭ����ͼ��ʾ���õ�ع���ʱ�ܷ�ӦʽΪ2Al+3Ag2O+2NaOH�T2NaAlO2+6Ag+H2O��������˵��������ǣ�������
Al-Ag2O�����һ�ֿ�����ˮ�¶�����������Դ����ԭ����ͼ��ʾ���õ�ع���ʱ�ܷ�ӦʽΪ2Al+3Ag2O+2NaOH�T2NaAlO2+6Ag+H2O��������˵��������ǣ�������| A�� | ����ʱ����������ԭ��Ӧ | |
| B�� | ���缫������1.08 g Agʱ����·��ת�Ƶĵ���Ϊ0.01 mol | |
| C�� | Al�缫�ķ�ӦʽΪAl-3e-+4OH-=AlO2-+2H2O | |
| D�� | ����ʱ���Һ�е�Na+����Ĥ����Al�缫�ŵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
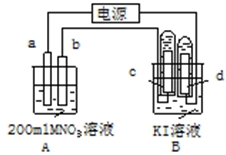 ��ͼװ���У�b�缫�ý���M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݣ��Իش�
��ͼװ���У�b�缫�ý���M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݣ��Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
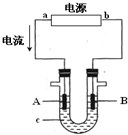 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ͭʱ��ͭ����������ͭ������ | |
| B�� | ���MgCl2������Һ�����Ƶý���þ | |
| C�� | �����ĸ�ʴͨ��Ϊ�绯ѧ��ʴ���ø�ʴ�����и�����ӦΪ��Fe-3e-�TFe3+ | |
| D�� | �ȼҵ�ͽ����Ƶ�ұ�����õ���NaCl��������Ӧ���ǣ�2Cl--2e-�TCl2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ������ƿǰ������Ƿ�©ˮ | |
| B�� | ����ƿ������ˮϴ�������ô�����Һ��ϴ | |
| C�� | �Ǻ�ƿ������ʳָ��סƿ��������һֻ��ָ��סƿ�ף�������ƿ��ת��ҡ������ | |
| D�� | ����ƿ���ܳ��ڴ�����ƺõ���Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ZR��ɵĻ�����Ϊ���ӻ����� | B�� | ����������R��X | ||
| C�� | X��Yֻ�ܹ����ڹ��ۻ������� | D�� | ԭ�Ӱ뾶Z��R��Y��X |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���������� | ||
| A | ij��Һ�м������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ��Һ��һ������CO32- |
| B | ��ɰֽ��ĥ�����������ھƾ������������գ��ۻ�������СҺ�ε���ʽ�������� | Al���۵�ͣ�����ʱAl�ۻ� |
| C | �ýྻ��˿պȡij��Һ�ڻ��������գ�����ʻ�ɫ | ��Һ����Na+���ަ�+ |
| D | ��ij��Һ���ȼӼ���KSCN��Һ�������������ٵμ���ˮ����Һ��� | ��Һ��һ������Fe2+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
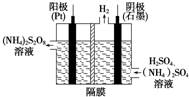 ��1����������立�����ͼ��ʾ����Ŀǰ�����е��Ʊ�H2O2�ķ���������⺬H2SO4����NH4��2SO4����Һ��ȡ��NH4��2S2O8��������ˮ��Ӧ���ɵ�H2O2�ͣ�NH4��2SO4��
��1����������立�����ͼ��ʾ����Ŀǰ�����е��Ʊ�H2O2�ķ���������⺬H2SO4����NH4��2SO4����Һ��ȡ��NH4��2S2O8��������ˮ��Ӧ���ɵ�H2O2�ͣ�NH4��2SO4���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com