�⣺��1���ܡ��������ˣ�ϴ�ӣ�����������ϸ��
�ʴ�Ϊ�����
�ݡ��ɹ������̿�֪���е�CuO���ܺ�������ͭ�����������ʣ�ȡ���ϴ��Һ������BaCl
2��Һ�������ǣ�֤��������ϴ����
�ʴ�Ϊ��ȡ���ϴ��Һ������BaCl
2��Һ�������ǣ�֤��������ϴ����
��2�����ɱ�����Ϣ��֪��˫��ˮ������һ�����Ƚϴ���������ͬʱ�������ɵ��������Խ�ࣨ�������ͬ�����������Ҫ��ʱ��Խ�̣���������Խǿ������Ӧ����������Ϊ��ͬʱ���ڲ��������������������ͬ�����������Ҫ��ʱ�䣩��
�ʴ�Ϊ����ͬʱ���ڲ��������������������ͬ�����������Ҫ��ʱ�䣩��
�ڴ����ڷ�Ӧǰ��ѧ���ʲ��䣬�������䣮�ʻ���CuO�������ڷ�Ӧǰ���Ƿ����ı䣮
�ʴ�Ϊ��CuO�������ڷ�Ӧǰ���Ƿ����ı䣮
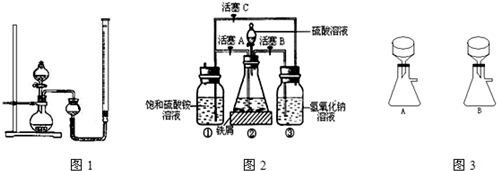
��2����װ��ͼ��֪������������ͨ��������Ӧ�رջ���A������B��C��
�������Һ�е��ܽ�O
2��������Һ���ϲ���O
2��Fe
2+�ױ�����ΪFe
3+��ͨ�����������������Һ�е��ܽ�O
2��������Һ���ϲ���O
2����ֹFe
2+������ΪFe
3+��
�ʴ�Ϊ��A��B��C�������������Һ�е��ܽ�O
2��������Һ���ϲ���O
2����ֹFe
2+������ΪFe
3+��
��3������©���ľ��¿�б��Ӧ�����ƿ��֧����ԣ������ڼ�ѹ���ˣ���A��ȷ��B����
��ѡ��A��
��4����24.50g��Ʒ��Fe
2+�����ʵ���Ϊxmol����
5Fe
2+������������MnO
4-��
5 1
xmol 0.01L��0.1000mol/L��10
����x=
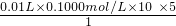
=0.05mol��
����24.50g��Ʒ�У�NH
4��
2SO
4?FeSO
4?6H
2O��Ϊ0.05mol��
����24.50g��Ʒ�У�NH
4��
2SO
4?FeSO
4?6H
2O������Ϊ0.05mol��392g/mol=19.6g��
����24.50g��Ʒ�У�NH
4��
2SO
4?FeSO
4?6H
2O����������Ϊ

��100%=80%��
�ʴ�Ϊ��80%��
��������1���ܡ��������ˣ�ϴ�ӣ�����������ϸ��
�ݡ��ɹ������̿�֪���е�CuO���ܺ�������ͭ�����������ʣ���BaCl
2��Һ����ϴ�Ӻ����Һ���Ƿ����������
��2�����ɱ�����Ϣ��֪��˫��ˮ������һ�����Ƚϴ���������ͬʱ�������ɵ��������Խ�ࣨ�������ͬ�����������Ҫ��ʱ��Խ�̣���������Խǿ��
�ڴ����ڷ�Ӧǰ��ѧ���ʲ��䣬�������䣮
��2����װ��ͼ��֪�������ڵij����ܲ���Һ�����£�����ֻ�ܴ������ڵĶ̵��ܳ��������������ۼ����������У�
ͨ�������ž�װ���ڿ�������ֹFe
2+������
��3������©���ľ��¿�б�������ƿ��֧����ԣ�
��4�����ݹ�ϵʽ5Fe
2+��MnO
4-����24.50g��Ʒ��Fe
2+�����ʵ������ɣ�NH
4��
2SO
4?FeSO
4?6H
2O��֪Fe
2+�����ʵ�������Ī���ε����ʵ������ٸ���m=nM����Ī���ε�������������������������㣮
������������Ī���ε��Ʊ�Ϊ���壬�������ʷ����ᴿ���й�ʵ�������ʵ��ԭ����װ�õ�������������ۡ��Դ�������̽��ʵ������⡢������ԭ��Ӧ�ζ�Ӧ���Լ��㡢�Լ������龳���ۺ�����֪ʶ�������������ȣ���Ŀ��һ�����Ѷȣ���ѧ��������ʵ�Ļ���֪ʶ���������֪ʶ��������������ע�⣨4���м��㣬��������ȡ��Һ�����Ϊ�״��㣮

 +5Fe2++8H+=Mn2++5Fe3++4H2O
+5Fe2++8H+=Mn2++5Fe3++4H2O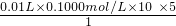 =0.05mol��
=0.05mol�� ��100%=80%��
��100%=80%��


