
��֪��Ӧ�٣�CO(g)��CuO(s)CO2(g)��Cu(s)�ͷ�Ӧ�ڣ�H2(g)��CuO(s)Cu(s)��H2O(g)����ͬ��ij�¶��µ�ƽ�ⳣ���ֱ�ΪK1��K2�����¶��·�Ӧ�ۣ�CO(g)��H2O(g)CO2(g)��H2(g)��ƽ�ⳣ��ΪK��������˵����ȷ����(����)
A����Ӧ�ٵ�ƽ�ⳣ��K1��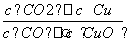
B����Ӧ�۵�ƽ�ⳣ��K��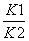
C�����ڷ�Ӧ�ۣ�����ʱ���¶����ߣ�H2Ũ�ȼ�С����÷�Ӧ���ʱ�Ϊ��ֵ
D�����ڷ�Ӧ�ۣ����º����£�����ѹǿ��H2Ũ��һ����С
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ᴿ�������ʣ������ڵ����������ʣ�����ѡ�õij����Լ��ͷ��뷽������ȷ���ǣ� ��
| ���ᴿ������ | �����Լ� | ���뷽�� | |
| A | �廯����Һ��NaI�� | ��ˮ��CCl4 | ��ȡ����Һ |
| B | �Ȼ����Һ��FeCl3�� | ����������Һ | ���� |
| C | ����������NO�� | ���� | ͨ�����O2 |
| D | ̼��������Һ��̼���ƣ� | ������̼ | ����Һ��ͨ�����CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����ܱ������У����淴ӦA(s)B��C(g) ����H��Q kJ/mol (Q>0)�ﵽƽ�⡣��С������������´ﵽƽ��ʱ��C(g)��Ũ������С���ǰ��ƽ��Ũ����ȡ����·�����ȷ����(����)
A������B��״ֻ̬��Ϊ��̬��Һ̬
B��ƽ��ʱ����λʱ����n(A)������n(C)������1��1
C������������䣬��ƽ����ϵ�м���B��ƽ��������淴Ӧ�����ƶ�
D������ʼʱ�������м���1 mol B��1 mol C���ﵽƽ��ʱ�ų�����Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��Ӧ4A(s)��3B(g)2C(g) ��D(g)����2 min��B��Ũ�ȼ���0.6 mol/L���Դ˷�Ӧ���ʵı�ʾ��ȷ����(����)
��D(g)����2 min��B��Ũ�ȼ���0.6 mol/L���Դ˷�Ӧ���ʵı�ʾ��ȷ����(����)
A����A��ʾ�ķ�Ӧ������0.4 mol��(L��min)��1
B���ֱ���B��C��D��ʾ�ķ�Ӧ�������ֵ��3��2��1
C��2 minĩ�ķ�Ӧ������B��ʾ��0.3 mol��(L��min)��1
D��2 min�ڣ�v��(B)��v��(C)��ʾ�ķ�Ӧ���ʵ�ֵ������С��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2013������������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
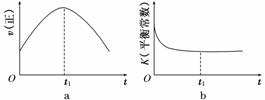
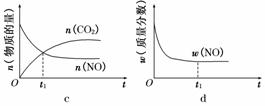
(1)����β����������Ҫԭ��Ϊ2NO(g)��2CO(g) 2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯������ͼ��ʾ��
2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯������ͼ��ʾ��
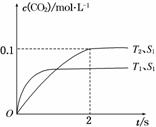
�ݴ��жϣ�
�ٸ÷�Ӧ�Ħ�H________0(�>����<��)
����T2�¶��£�0��2 s�ڵ�ƽ����Ӧ����v(N2)��______________________��
�۵��������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2������ͼ�л���c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����________(�����)��
(2)ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ� ���������������������CH4����ԭNOx�������������������Ⱦ��
���������������������CH4����ԭNOx�������������������Ⱦ��
���磺CH4(g)��2NO2(g)===N2(g)��CO2(g)��2H2O(g)����H1����867 kJ/mol
2NO2(g)N2O4(g)����H2����56 .9 kJ/mol
.9 kJ/mol
д��CH4(g)����ԭN2O4(g)����N2(g)��CO2(g)��H2O(g)���Ȼ�ѧ����ʽ��________________________________________________________________________��
�ڽ�ȼú�����Ķ�����̼�������ã��ɴﵽ��̼�ŷŵ�
Ŀ�ġ���ͼ��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪ__________________________��
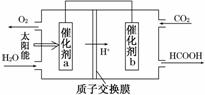
�۳����£�0.1 mol��L��1��HCOONa��ҺpHΪ10����HCOOH�ĵ��볣��Ka��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʱ��M(OH)2(s)M2��(aq)��2OH��(aq)��Ksp��a��c(M2��)��b mol��L��1ʱ����Һ��pH����(����)
A.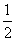 lg(
lg(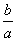 ) B.
) B.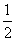 lg(
lg(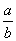 )
)
C��14��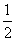 lg(
lg(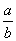 ) D��14��
) D��14��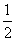 lg(
lg(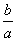 )
)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��֤��һˮ�ϰ�(NH3��H2O)��������ʣ��ס��ҡ������˷ֱ�ѡ�������Լ�����ʵ�飺0.010 mol��L��1��ˮ��0.1 mol��L��1NH4Cl��Һ��NH4Cl���塢��̪�Լ���pH��ֽ������ˮ��
(1)����pH��ֽ���0.010 mol��L��1��ˮ��pHΪ10�����϶�һˮ�ϰ���������ʣ�����Ϊ��һ�����Ƿ���ȷ��________(��ǡ���)��˵������_______________________
________________________________________________________________________��
(2)��ȡ��10 mL 0.010 mol��L��1��ˮ����pH��ֽ�����pH��a��Ȼ��������ˮϡ����1 000 mL������pH��ֽ�����pHΪb����Ҫȷ��NH3��H2O��������ʣ���a��bֵӦ����ʲô��ϵ��________(�á���ʽ������ʽ����ʾ)��
(3)��ȡ��10 mL 0.010 mol��L��1��ˮ������2�η�̪��Һ���Էۺ�ɫ���ټ���NH4Cl������������ɫ��dz������Ϊ��һ�����ܷ�֤��NH3��H2O��������ʣ���˵��ԭ��
________________________________________________________________________
________________________________________________________________________��
(4)����������ṩ���Լ��������һ�������ּ��ķ���֤�� NH3��H2O��������ʣ�
NH3��H2O��������ʣ�
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʣ����ռ���壻��ͭ˿��
��NH3����ϡ����ݶ�����̼���壻��ʳ��ˮ����̼���Ʒ�ĩ����ƾ����������Ȼ��ƣ���������������գ�
(1)����״̬�µ����ʿɵ������___________________________________��
(2)���ڵ���ʵ���____________________________________________��
(3)���ڷǵ���ʵ���_____________________________________��
(4)���ڵ���ʣ���������״̬�²��ܵ������____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com