������ʵ������У���������
AC
AC
A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ���ʹҺ���¶�Ѹ������170��
B����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����ã���Һ��ֲ�μ���������Һ
C����ͭ˿�������״���ھƾ����ϼ��ȱ�ں�����������ˮ�Ҵ��У�����Ҵ�����Ϊ��ȩ��ʵ��
D�������еμ�����ϡ��ˮ���������������鱽��
E����ҵ�ƾ���ȡ��ˮ�ƾ�ʱ���ȼ���ʯ��Ȼ������������뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ�¶�
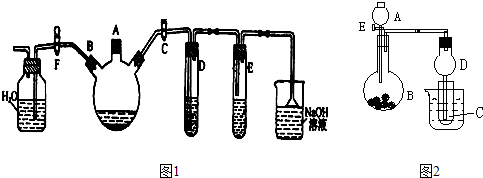
��ʵ������ͼ1��ʾװ���Ʊ��屽������֤�÷�Ӧ��ȡ����Ӧ��
��1���ر�F��������C��������װ��������������ƿ����A�ڼ��������壬�ټ���������м����סA�ڣ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��
��
��2��D�Թ���װ����
CCl4
CCl4
����������
���ջӷ����ı���������
���ջӷ����ı���������
��
��3��E�Թ���װ����
AgNO3��Һ
AgNO3��Һ
��E�Թ��ڳ��ֵ�����Ϊ
���ܿڲ����������������ɵ���ɫ����
���ܿڲ����������������ɵ���ɫ����
��
��4����������ƿ�еķ�Ӧ��������ʱ����ʱ�������Լ��٣�����F�������ر�C���������Կ�����������
ˮ������������ƿ��
ˮ������������ƿ��
��
��5����һ���õ����屽��Ҫ�����²������ƣ�
a���� bˮϴ�� c�ø������� d 10%NaOH��Һϴ�ӣ� eˮϴ
��ȷ�IJ���˳����
bdeca����edbca��
bdeca����edbca��
������ͼ2��ʾװ�ý���ʵ�飬��A��μ���B�У�
��1����BΪNa
2CO
3��ĩ��CΪC
6H
5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ����Թ�C�л�ѧ��Ӧ�����ӷ���ʽ��
��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�����
��Һ�ɻ��DZ����
��Һ�ɻ��DZ����
��
��2����B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E��Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��
Ũ��ˮ
Ũ��ˮ
�������ƣ���C��
AgNO3
AgNO3
���ѧʽ��������ȩ�Ļ�Ϻ���Һ�з�Ӧ�Ļ�ѧ����ʽ��
CH
3CHO+2Ag��NH
3��
2OH

CH
3COONH
4+H
2O+2Ag��+3NH
3CH
3CHO+2Ag��NH
3��
2OH

CH
3COONH
4+H
2O+2Ag��+3NH
3������D�ڴ�ʵ���е�������
��ֹ����
��ֹ����
��



 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�


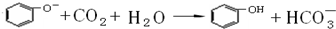
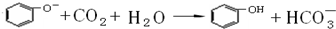
 CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3 CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3