
ÓŠ»śĪļGŹĒŅ»ÖÖŹ³Ę·ĻćĮĻ£¬ĘäĻćĘųĒæ¶ČĪŖĘÕĶØĻćĮĻµÄ3”«4±¶£¬ÓŠ»śĪļGµÄŗĻ³ÉĀ·ĻßČēĻĀ£ŗ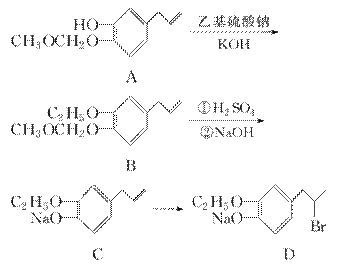
(1)øĆĻćĮĻ³¤ĘŚ±©Ā¶ÓŚæÕĘųÖŠŅ×±äÖŹ£¬ĘäŌŅņŹĒ__________________________________________________________________________________________________________________________________________”£
(2)Š“³öAÖŠŗ¬Ńõ¹ŁÄÜĶŵÄĆū³Ę£ŗ________£¬ÓÉCµ½ DµÄ·“Ó¦ĄąŠĶĪŖ________”£
(3)ÓŠ»śĪļEµÄ½į¹¹¼ņŹ½ĪŖ________________________________________________________________________”£
(4)ÓŠ»śĪļGĶ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄĶ¬·ÖŅģ¹¹ĢåÓŠ____________ÖÖ”£
¢ŁÓėFeCl3ČÜŅŗ·“Ó¦ĻŌ×ĻÉ«£»
¢ŚæÉ·¢ÉśĖ®½ā·“Ó¦£¬ĘäÖŠŅ»ÖÖĖ®½ā²śĪļÄÜ·¢ÉśŅų¾µ·“Ó¦£»
¢Ū·Ö×ÓÖŠÓŠ4ÖÖ²»Ķ¬»Æѧ»·¾³µÄĒā”£
½āĪö””ÕĘĪÕĻŽÖĘĢõ¼žĻĀĶ¬·ÖŅģ¹¹ĢåµÄŹéŠ“·½·Ø”£(1)¹Ū²ģGÖŠ½į¹¹£¬æÉÖŖĘäÖŠŗ¬ÓŠµÄ¹ŁÄÜĶÅÓŠ·ÓōĒ»ł”¢Č©»ł”¢ĆŃ¼ü£¬ĘäÖŠ·ÓōĒ»ł”¢Č©»łŅ×±»æÕĘųŃõ»Æ”£(2)AÖŠŗ¬Ńõ¹ŁÄÜĶÅĪŖĆŃ¼ü”¢·ÓōĒ»ł”£¶ŌÓŚC”¢D½į¹¹æÉŅŌ·¢ĻÖ£¬CÖŠµÄC===CÓėHBr¼Ó³ÉÉś³ÉD£¬C”śDµÄ·“Ó¦ĄąŠĶĪŖ¼Ó³É·“Ó¦”£(3)FÓÉE¾O3Ńõ»Æ»ńµĆ£¬½įŗĻŠÅĻ¢£¬—CHOĄ“×ŌĢ¼Ģ¼Ė«¼üµÄ³ōŃõŃõ»Æ£¬EÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü”£ŌŁÓÉDµÄ½į¹¹ÖŠŗ¬ÓŠäåŌ×Ó£¬äåŌ×Ó¾ĻūČ„·“Ó¦æÉŅŌ»ńµĆĖ«¼ü£¬½įŗĻFÖŠµÄ—CHO£¬ÅŠ¶ĻĢ¼Ģ¼Ė«¼üµÄĪ»ÖĆ”£(4)G½į¹¹ÖŠ³ż±½»·Ķā£¬»¹ÓŠ3øöĢ¼Ō×Ó”¢3øöŃõŌ×Ó”¢1øö²»±„ŗĶ¶Č”£ÓöFeCl3ČÜŅŗĻŌ×ĻÉ«£¬ĖµĆ÷ŗ¬ÓŠ·ÓōĒ»ł£¬æÉŅŌ·¢ÉśĖ®½ā£¬æÉÄÜŗ¬ÓŠõ„µÄ½į¹¹(Ö»ÓŠC”¢H”¢OČżÖÖŌŖĖŲ)£¬Ė®½ā²śĪļÄÜ·¢ÉśŅų¾µ·“Ó¦£¬ĖµĆ÷Ė®½ā²śĪļÖŠÓŠHCOOH£¬ĖµĆ÷Ķ¬·ÖŅģ¹¹ĢåÖŠÓŠ¼×Ėįõ„½į¹¹£¬¼“HCOO—”£·ÓōĒ»ł”¢HCOO—£¬ÓƵōĮĖ1øöĢ¼Ō×Ó”¢3øöŃõŌ×Ó”¢1øö²»±„ŗĶ¶Č£¬»¹ÓŠ2øö±„ŗĶĢ¼Ō×Ó”£½įŗĻ4ÖÖ²»Ķ¬»·¾³µÄĒāŌ×Ó£¬Š“³ö½į¹¹£ŗ
 ӣ
ӣ
“š°ø””(1)ĻćĮĻ·Ö×ÓÖŠŗ¬ÓŠ·ÓōĒ»łŗĶČ©»ł£¬Ņ×±»Ńõ»Æ
(2)ĆŃ¼ü”¢·ÓōĒ»ł””¼Ó³É·“Ó¦
(3) 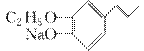 ””(4)2
””(4)2


 ŗ®¼ŁĄÖŌ°±±¾©½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
ŗ®¼ŁĄÖŌ°±±¾©½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĒóĖćĻĀĮŠ³£ĪĀĻĀČÜŅŗÖŠÓÉH2OµēĄėµÄc(H£«)ŗĶc(OH£)”£
(1)pH£½2µÄH2SO4ČÜŅŗ
c(H£«)£½__________£¬c(OH£)£½__________”£
(2)pH£½10µÄNaOHČÜŅŗ
c(H£«)£½__________£¬c(OH£)£½__________”£
(3)pH£½2µÄNH4ClČÜŅŗ
c(H£«)£½__________”£
(4)pH£½10µÄNa2CO3ČÜŅŗ
c(OH£)£½__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚAl2(SO4)3ŗĶMgSO4µÄ»ģŗĻČÜŅŗÖŠ£¬µĪ¼ÓNaOHČÜŅŗ£¬Éś³É³ĮµķµÄĮæÓėµĪČėNaOHČÜŅŗµÄĢå»ż¹ŲĻµČēÓŅĶ¼ĖłŹ¾£¬ŌņŌ»ģŗĻŅŗÖŠAl2(SO4)3ÓėMgSO4µÄĪļÖŹµÄĮæÅضČÖ®±ČĪŖ(””””)”£
A£®6”Ć1 B£®3”Ć1
C£®2”Ć1 D£®1”Ć2
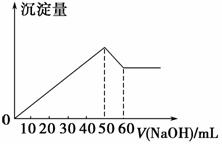
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)ŅŃÖŖ£ŗ
¢ŁFe(s)£«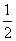 O2(g)===FeO(s)
O2(g)===FeO(s)
¦¤H1£½£272.0 kJ·mol£1£»
¢Ś2Al(s)£«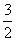 O2(g)===Al2O3(s)
O2(g)===Al2O3(s)
¦¤H2£½£1 675.7 kJ·mol£1”£
AlŗĶFeO·¢ÉśĀĮČČ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ_______________________________________________________________
_______________________________________________________________ӣ
ijĶ¬Ń§ČĻĪŖ£¬ĀĮČČ·“Ó¦æÉÓĆÓŚ¹¤ŅµĮ¶Ģś£¬ÄćµÄÅŠ¶ĻŹĒ________(Ģī”°ÄÜ”±»ņ”°²»ÄÜ”±)£¬ÄćµÄĄķÓÉŹĒ___________________________________________
________________________________________________________________ӣ
(2)·“Ó¦ĪļÓėÉś³ÉĪļ¾łĪŖĘųĢ¬µÄijæÉÄę·“Ó¦ŌŚ²»Ķ¬Ģõ¼žĻĀµÄ·“Ó¦Ąś³Ģ·Ö±šĪŖA”¢B£¬ČēĶ¼ĖłŹ¾”£
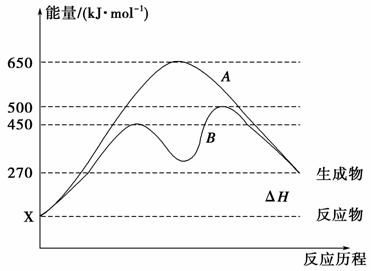
¢Ł¾ŻĶ¼ÅŠ¶ĻøĆ·“Ó¦ŹĒ________(Ģī”°Īü”±»ņ”°·Å”±)ČČ·“Ó¦£¬µ±·“Ó¦“ļµ½Ę½ŗāŗó£¬ĘäĖūĢõ¼ž²»±ä£¬ÉżøßĪĀ¶Č£¬·“Ó¦ĪļµÄ×Ŗ»ÆĀŹ½«________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
¢ŚĘäÖŠBĄś³Ģ±ķĆ÷“Ė·“Ó¦²ÉÓƵÄĢõ¼žĪŖ________(Ģī×ÖÄø)”£
A£®ÉżøßĪĀ¶Č B£®Ōö“ó·“Ó¦ĪļµÄÅضČ
C£®½µµĶĪĀ¶Č D£®Ź¹ÓĆ“ß»Æ¼Į
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĆŌµüĻćĖįŹĒ“Ó·ä»ØŹōÖ²ĪļÖŠĢįČ”µĆµ½µÄĖįŠŌĪļÖŹ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼ĖłŹ¾”£ĻĀĮŠŠšŹöÕżČ·µÄŹĒ (””””)”£
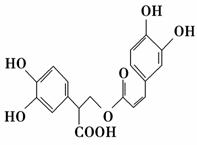
A£®ĆŌµüĻćĖįÓėäåµ„ÖŹÖ»ÄÜ·¢ÉśČ”“ś·“Ó¦
B£®1 molĆŌµüĻćĖį×ī¶ąÄÜŗĶ9 molĒāĘų·¢Éś¼Ó³É·“Ó¦
C£®ĆŌµüĻćĖįæÉŅŌ·¢ÉśĖ®½ā·“Ó¦”¢Č”“ś·“Ó¦ŗĶõ„»Æ·“Ó¦
D£®1 molĆŌµüĻćĖį×ī¶ąÄÜÓė5 mol NaOH·¢Éś·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Č”Ņ»Š”æ齚ŹōÄĘ£¬·ÅŌŚČ¼ÉÕ³×Ąļ¼ÓČČ£¬ĻĀĮŠŹµŃéĻÖĻóĆčŹöÕżČ·µÄŹĒ________(ĢīŠņŗÅ)”£
¢Ł½šŹōĻČČŪ»Æ””¢ŚŌŚæÕĘųÖŠČ¼ÉÕ£¬·Å³ö»ĘÉ«»š»Ø””¢ŪČ¼ÉÕŗóµĆ°×É«¹ĢĢå””¢ÜČ¼ÉÕŹ±»šŃęĪŖ»ĘÉ«””¢ŻČ¼ÉÕŗóÉś³Éµ»ĘÉ«¹ĢĢåĪļÖŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÅŠ¶ĻÕżĪó£¬ÕżČ·µÄ»®”°”Ģ”±£¬“ķĪóµÄ»®”°”Į”±
(1)Na2O2ÓėNa2OÖŠ¾łÖ»“ęŌŚĄė×Ó¼ü(””””)
(2)Na2O2ÓėNa2O¾§ĢåÖŠŃōĄė×ÓÓėŅõĄė×ÓµÄøöŹż±Č¾łĪŖ2”Ć1(””””)
(3)2Na2O2£«2H2O===4NaOH£«O2”ü
H2O¼Č²»ŹĒŃõ»Æ¼ĮŅ²²»ŹĒ»¹Ō¼Į(””””)
(4)Na2O”¢Na2O2×é³ÉŌŖĖŲĻąĶ¬£¬ÓėCO2·“Ó¦²śĪļŅ²ĻąĶ¬(””””)
(5)Na2O2µÄµē×ÓŹ½ĪŖNa
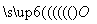 ·
· ·
·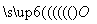
 Na(””””)
Na(””””)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾Ż¼ī½šŹōµÄŠŌÖŹ¹ęĀÉĖ¼æ¼ĻĀĮŠĪŹĢā”£
(1)ÄĘÓėŃõĘų·“Ó¦ÓŠNa2O”¢Na2O2Į½ÖÖŃõ»ÆĪļÉś³É£¬ĘäĖū¼ī½šŹōµ„ÖŹÓėŃõĘų·“Ó¦Ņ²Ö»Éś³ÉĮ½ÖÖĄąĖʵÄŃõ»ÆĪļĀš£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
1)ŅŌĻĀĮŠ³öµÄŹĒŅ»Š©Ō×ÓµÄ2pÄܼ¶ŗĶ3dÄܼ¶ÖŠµē×ÓÅŲ¼µÄĒéæö”£ŹŌÅŠ¶Ļ£¬ÄÄŠ©Ī„·“ĮĖÅŻĄū²»ĻąČŻŌĄķ________£¬ÄÄŠ©Ī„·“ĮĖŗéĢŲ¹ęŌņ____________________”£

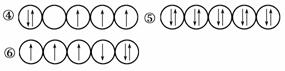
(2)ijŌŖĖŲµÄ¼¤·¢Ģ¬(²»ĪȶØדĢ¬)Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s13p33d2£¬ŌņøĆŌŖĖŲ»łĢ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ____________________________£»Ęä×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļµÄ»ÆѧŹ½ŹĒ________”£
(3)½«ĻĀĮŠ¶ąµē×ÓŌ×ÓµÄŌ×Ó¹ģµĄ°“¹ģµĄÄÜĮæÓɵĶµ½øßĖ³ŠņÅÅĮŠ£ŗ2s””3d””4s””3s””4p””3p
¹ģµĄÄÜĮæÓɵĶµ½øßÅÅĮŠĖ³ŠņŹĒ____________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com