
Cl
2��һ�ֳ��õ�����ˮ��������������أ�K
2FeO
4����һ�����͡���Ч�������ɫˮ����������Cl
2��O
2��ClO
2��KMnO
4�����Ը�ǿ��������Ⱦ����ҵ�������Ƶø������ƣ�Ȼ���ڵ����£������������Һ�м���KOH�����ͣ�ʹ�������������
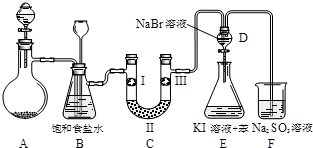
����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���
��1��д��A�з��������ӷ�Ӧ����ʽ��
MnO
2+4H
++2Cl
-Mn
2++Cl
2��+2H
2O
MnO
2+4H
++2Cl
-Mn
2++Cl
2��+2H
2O
��
��2��װ��B�б���ʳ��ˮ��������
��ȥCl2�е�HCl
��ȥCl2�е�HCl
��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����
��ƿ��Һ���½�������©����Һ������
��ƿ��Һ���½�������©����Һ������
��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���
d
d
��
|
a |
b |
c |
d |
| �� |
�������ɫ���� |
�������ɫ���� |
ʪ�����ɫ���� |
ʪ�����ɫ���� |
| �� |
��ʯ�� |
�轺 |
Ũ���� |
��ˮ�Ȼ��� |
| �� |
ʪ�����ɫ���� |
ʪ�����ɫ���� |
�������ɫ���� |
�������ɫ���� |
II����1���ɷ��Ʊ�������ص���Ҫ��ӦΪ��2FeSO
4+6Na
2O
2�T2Na
2FeO
4+2Na
2O+2Na
2SO
4+O
2��
�ٸ÷�Ӧ�е���������
Na2O2
Na2O2
����ԭ����
Na2O2��FeSO4
Na2O2��FeSO4
��ÿ����1mol Na
2FeO
4ת��
5NA
5NA
�����ӣ�
�ڼ�Ҫ˵��K
2FeO
4��Ϊˮ������ʱ���������
������ؾ���ǿ�����ԣ���ɱ��������������������������ԭΪFe3+��Fe3+ˮ������Fe��OH��3����������ˮ���������ʶ�����
������ؾ���ǿ�����ԣ���ɱ��������������������������ԭΪFe3+��Fe3+ˮ������Fe��OH��3����������ˮ���������ʶ�����
��
��2��ʪ���Ʊ�������أ�K
2FeO
4���ķ�Ӧ��ϵ��������������Fe��OH��
3��ClO
-��OH
-��FeO
42-��Cl
-��H
2O��д������ƽʪ���Ƹ�����ص����ӷ�Ӧ����ʽ��
2Fe��OH��3+3C1O-+4OH-�T2FeO42-+3C1-+5H2O
2Fe��OH��3+3C1O-+4OH-�T2FeO42-+3C1-+5H2O
��


 MnCl2+Cl2��+2H2O����Ӧ�������Ȼ����ˮ������������ �����ʣ�װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��װ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������ʱB�еģ�ѹǿ����B�г���©����Һ���������γ�ˮ����
MnCl2+Cl2��+2H2O����Ӧ�������Ȼ����ˮ������������ �����ʣ�װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��װ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������ʱB�еģ�ѹǿ����B�г���©����Һ���������γ�ˮ���� MnCl2+Cl2��+2H2O����ȥCl2�е�HCl��B�г���©����Һ���������γ�ˮ����
MnCl2+Cl2��+2H2O����ȥCl2�е�HCl��B�г���©����Һ���������γ�ˮ����


 ��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᣮ
��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᣮ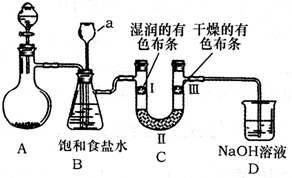
 Cl2��һ�ֳ��õ�����ˮ��������������أ�K2FeO4����һ�����͡���Ч�������ɫˮ����������Cl2��O2��ClO2��KMnO4�����Ը�ǿ��������Ⱦ����ҵ�������Ƶø������ƣ�Ȼ���ڵ����£������������Һ�м���KOH�����ͣ�ʹ�������������
Cl2��һ�ֳ��õ�����ˮ��������������أ�K2FeO4����һ�����͡���Ч�������ɫˮ����������Cl2��O2��ClO2��KMnO4�����Ը�ǿ��������Ⱦ����ҵ�������Ƶø������ƣ�Ȼ���ڵ����£������������Һ�м���KOH�����ͣ�ʹ�������������
 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O