
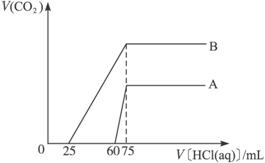
�Իش��������⣺
��1������A������ԭ��Һ��ͨ��CO2��������Һ�е�����Ϊ��д��ѧʽ��_____________���������ʵ����ʵ���֮��Ϊ_____________����ϡ�ͺ����Һ����μ���0.1 mol��L-1�����ᣬ����CO2��������ֵΪ_____________mL��
��2������B������ԭ��Һ��ͨ��CO2��������Һ�е�����Ϊ��д��ѧʽ��_____________����ϡ�ͺ����Һ����μ���0.1 mol��L-1�����ᣬ����CO2������ֵ_____________mL��
��3��ԭNaOH��Һ�����ʵ���Ũ��Ϊ_____________mol��L-1��
��1��NaOH��Na2CO3 3��1 33.6��2��Na2CO3��NaHCO3 112 ��3��0.75
��������1��A���߲���CO2��n(HCl)=��75 mL-60 mL����10-3L��mL-1��0.1 mol��L-1=0.001 5 mol������CO2��B�٣���A��ͨ��CO2���㣬��������巴Ӧ��Ϊ
NaHCO3+HCl====NaCl+CO2��+H2O����AΪNaOH��Na2CO3�Ļ�����
Na2CO3 + HCl ==== NaHCO3 +H2O
0.001 5 mol 0.001 5 mol 0.001 5 mol
NaHCO3 + HCl ==== NaCl+H2O
0.001 5 mol 0.001 5 mol
���к�NaOH����HClΪ75 mL��10-3 L��mL-1��0.1 mol��L-1-(0.001 5 mol��2)=0.004 5 mol
![]() =3��1,V(CO2)=33.6 mL
=3��1,V(CO2)=33.6 mL
(2)B���߷�����ӦNaHCO3+HCl====NaCl+CO2��+H2O������n(HCl)=50 mL��10-3L��mL-1��0.1 mol��L-1=0.005 mol����BΪNa2CO3��NaHCO3�Ļ�����
Na2CO3 + HCl ==== NaCl+NaHCO3
0.0025 mol 0.0025 mol
��n��NaHCO3��=0.002 5 mol,V(CO2)=112 mL����AΪ10 mL��n��Na+��=0.004 5 mol+0.001 5 mol��2=0.007 5 mol
c=![]() =0.75 mol��L-1
=0.75 mol��L-1



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȡ25.00 mL����Һ�������л����μ�����Һ15.00 mL�����ռ���������̼����224 mL��
����ȡ15.00 mL����Һ�������л����μӼ���Һ25.00 mL�����ռ���������̼����112 mL��
��������������ѻ���Ϊ��״�������������������ʵ��������գ�
��1��д���������������漰��Ӧ�����ӷ���ʽ����_______________����______________����______________��
��2������______________������Һ�����ʵ���Ũ��Ϊ______________������Һ�����ʵ���Ũ��Ϊ______________����������̼����Һ�е������ܽ���Բ��ƣ�
��3����n mL�ļ���Һ������������Һ�����ֿ��ܵķ�ʽ��ϣ��������������ΪV mL����״��������V��ȡֵ��ΧΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ϻ��з���������4�µ��в��ԣ���ģ����ѧ�Ծ� ���ͣ������
���л�ѧѧϰ�Σ����Ĵ�������ӦҲ����ij����M�����ԭ��������100������������������MԪ�ص�������һ������������NH3��±���ӵȰ�ij�̶��������ν�ϳ��ȶ�����������ӣ�������ԭ���ţ���
��1��150�桢��ѹ�£�6.08g����������0.6mol HCl�����ַ������ֽⷴӦ����������ʣ�࣬�����Ϊ0.48mol���ý���M�����ԭ������Ϊ___________��
��������Ӧ���ù�������ˮ���50.0mL��Һ������Һ�����ʵ����ʵ���Ũ��Ϊ
_______mol��L-1��
��2��ȡ��1����������Һ12.5mL��ϡ����25mL������ͨ��2688mL����������£�����һ�������·�Ӧǡ����ȫ���õ�����B��Ħ������Ϊ260.5g/mol����������1.5mol/L��AgNO3��Һ�ζ����ﵽ�յ�ʱ����ȥAgNO3��Һ40.0mL����BͶ������ռ���Һ�У�δ����NH3���ݳ�����B�Ļ�ѧʽ�ɱ�ʾΪ ��
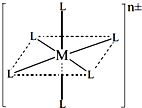
��3����֪����ͼ�У�L��λ����ȫ��ͬ��������һ��������[M(NH3)6-xClx]n����1��x��5����xΪ���������ṹ������ͼ��
����������ӹ���2�ֲ�ͬ�ṹ���������ӵ�ʽ��Ϊ ��
��4��һ�������£�3.04g�ý���������ǡ�ñ�ij��������Ч���൱��0.03mol O2���������ټ���0.05mol KCl����һЩ����մ�����K��M��Ԫ��ǡ����ȫ�γɺ������Σ�ʽ����360���������ڲ��������Ӹ�����Ϊ1:2������ε�ʽ���Լ����ʵ����ֱ�Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ϻ����ɽ���������ѧ����ĩ��һģ�����Ի�ѧ�Ծ��������棩 ���ͣ������
�̷���FeSO4��7H2O���ڻ�ѧ�ϳ���������ԭ������������ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷���
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ ����>��<��=��40%��
��2��ʵ��������20%�������ᣨ100�˷������ẬSO3 20�ˣ�����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL2mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS������������������λС������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com