
ЁОЬтФПЁПЃЈ1ЃЉМзДМЪЧвЛжжПЩдйЩњФмдДЃЌгУЭОЙуЗКЃЌвЛжжгЩМзДМЁЂбѕЦјвдМАЧПМюШмвКжЦГЩЕФаТаЭЪжЛњЕчГиЃЌШнСПДяЧтФјЕчГиЛђяЎЕчГиЕФ10БЖЁЃЛиД№ЯТСагаЙиЮЪЬтЃК
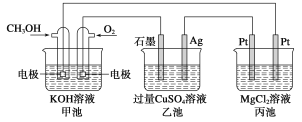
ШчЭМЫљЪОЃК
ЂйМзГиЭЈШыCH3OHЕФЕчМЋЗДгІЪНЮЊ__________ЃЛввГиЪЏФЋвЛМЋЕФЕчМЋЗДгІЪНЮЊ______ЃЛ
ЂкЗДгІвЛЖЮЪБМфКѓЃЌЯђввГижаМгШывЛЖЈСП________ФмЪЙCuSO4ШмвКЛжИДЕНдХЈЖШЃЛ
ЂлМзГижаЯћКФ280 mL(БъзМзДПіЯТ)O2ЃЌДЫЪББћГижаРэТлЩЯзюЖрВњЩњ__________gГСЕэЁЃ
ЃЈ2ЃЉЙЄвЕЩЯгавЛжжЩњВњМзДМЕФЗДгІЃКCO2(g) + 3H2(g)![]() CH3OH(g) + H2O(g) ЁїHЃН-49.0kJЁЄmol-1ЁЃдкФГЮТЖШЯТЃЌШнЛ§ОљЮЊ2LЕФAЁЂBСНИіШнЦїжаЃЌАДВЛЭЌЗНЪНЭЖШыЗДгІЮяЃЌБЃГжКуЮТКуШнЃЌО10УыжгКѓДяЕНЦНКтЃЌДяЕНЦНКтЪБЕФгаЙиЪ§ОнШчЯТБэЃК
CH3OH(g) + H2O(g) ЁїHЃН-49.0kJЁЄmol-1ЁЃдкФГЮТЖШЯТЃЌШнЛ§ОљЮЊ2LЕФAЁЂBСНИіШнЦїжаЃЌАДВЛЭЌЗНЪНЭЖШыЗДгІЮяЃЌБЃГжКуЮТКуШнЃЌО10УыжгКѓДяЕНЦНКтЃЌДяЕНЦНКтЪБЕФгаЙиЪ§ОнШчЯТБэЃК
ШнЦї | A | B |
ЗДгІЮяЭЖШыСП | 1mol CO2ЃЈgЃЉКЭ3mol H2ЃЈgЃЉ | 1mol CH3OHЃЈgЃЉКЭ1mol H2OЃЈgЃЉ |
CH3OHЃЈgЃЉХЈЖШЃЈmolL-1ЃЉ | c1 | c2 |
ЗДгІФмСПБфЛЏ | ЗХГі29.4kJ | ЮќЪеakJ |
ЂйДгЗДгІПЊЪМжСДяЕНЦНКтЪБЃЌAжагУCO2РДБэЪОЕФЦНОљЗДгІЫйТЪЮЊ______________ЃЛ
ЂкИУЮТЖШЯТЃЌЗДгІCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)ЕФЛЏбЇЦНКтГЃЪ§ЕФжЕЮЊ_____________ЃЛ
CH3OH(g)+H2O(g)ЕФЛЏбЇЦНКтГЃЪ§ЕФжЕЮЊ_____________ЃЛ
Ђлa=______________ЁЃ
ЂмЯТСаДыЪЉжаФмЪЙn(CH3OH)/n(CO2)діДѓЕФЪЧ____________ЁЃ
AЃЎЩ§ИпЮТЖШ BЃЎГфШыЧтЦј
CЃЎНЋЫЎеєЦјДгЬхЯЕжаЗжРы DЃЎгУИќгааЇЕФДпЛЏМС
ЁОД№АИЁПCH3OHЃ6e-ЃЋ8OH-ЃНCO32-ЃЋ6H2O4OH-4eЃНO2Ёќ+2H2OCuO1.45 g0.032.0819.6BC
ЁОНтЮіЁП
ЃЈ1ЃЉЂйМзГиЭЈШыCH3OHЕФЕчМЋЪЧИКМЋЃЌМзДМЪЇШЅЕчзгЃЌдкМюадЛЗОГЯТБфЮЊЬМЫсИљРызгЃЌЕчМЋЗДгІЪНЮЊCH3OHЃ6e-ЃЋ8OH-ЃНCO32-ЃЋ6H2OЃЛввГиЪЏФЋвЛМЋгыЕчдДЕФе§МЋЯрСЌЃЌзїбєМЋЃЌЧтбѕИљРызгЗХЕчЃЌЕчМЋЗДгІЪНЮЊ4OH-4eЃНO2Ёќ+2H2OЃЛе§ШЗД№АИЃКCH3OHЃ6e-ЃЋ8OH-ЃНCO32-ЃЋ6H2O ЃЛ4OH-4eЃНO2Ёќ+2H2OЁЃ
ЂкввГижабєМЋЧтбѕИљРызгЗХЕчЩњГЩбѕЦјЃЌвѕМЋЭРызгЗХЕчЩњГЩЭЃЌЕчНтзмЗДгІЮЊЃК2CuSO4+2H2O![]() 2Cu+2H2SO4+2O2ЁќЃЛвђДЫЗДгІвЛЖЮЪБМфКѓЃЌЯђввГижаМгШывЛЖЈСПCuOФмЪЙCuSO4ШмвКЛжИДЕНдХЈЖШЃЛе§ШЗД№АИЃКCuOЁЃ
2Cu+2H2SO4+2O2ЁќЃЛвђДЫЗДгІвЛЖЮЪБМфКѓЃЌЯђввГижаМгШывЛЖЈСПCuOФмЪЙCuSO4ШмвКЛжИДЕНдХЈЖШЃЛе§ШЗД№АИЃКCuOЁЃ
ЂлМзГижаЯћКФ280 mL(БъзМзДПіЯТ)O2ЃЌзЊвЦЕчзгЕФЮяжЪЕФСПЪЧ0.28ЁС4/22.4=0.05molЁЃЖшадЕчМЋЕчНтMgCl2ШмвКЩњГЩЧтбѕЛЏУОГСЕэЁЂТШЦјКЭЧтЦјЃЌРызгЗНГЬЪНЮЊMg2ЃЋЃЋ2H2OЃЋ2ClЃ![]() Cl2ЁќЃЋH2ЁќЃЋMg(OH)2Ё§ЃЌЫљвдИљОнЕчзгЪиКуПЩжЊЩњГЩЧтбѕЛЏУОЕФЮяжЪЕФСПЪЧ0.025molЃЌМДДЫЪББћГижаРэТлЩЯзюЖрВњЩњЧтбѕЛЏУОГСЕэЃК0.025molЁС58g/molЃН1.45gЃЛе§ШЗД№АИЃК1.45gЁЃ
Cl2ЁќЃЋH2ЁќЃЋMg(OH)2Ё§ЃЌЫљвдИљОнЕчзгЪиКуПЩжЊЩњГЩЧтбѕЛЏУОЕФЮяжЪЕФСПЪЧ0.025molЃЌМДДЫЪББћГижаРэТлЩЯзюЖрВњЩњЧтбѕЛЏУОГСЕэЃК0.025molЁС58g/molЃН1.45gЃЛе§ШЗД№АИЃК1.45gЁЃ
ЃЈ2ЃЉЂй1molЖўбѕЛЏЬМЭъШЋЗДгІЗХГі49.0kJШШСПЃЌЕБЗХГі29.4kJШШСПЪБЃЌдђВЮМгЗДгІЕФnЃЈCO2ЃЉ=29.4ЁС1/49=0.6molЃЌCO2ЕФЦНОљЗДгІЫйТЪv=![]() =0.6/(2ЁС10)==0.03mol/ЃЈLSЃЉе§ШЗД№АИЃК0.03ЁЃ
=0.6/(2ЁС10)==0.03mol/ЃЈLSЃЉе§ШЗД№АИЃК0.03ЁЃ
Ђк CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
Ц№ЪМСП 1 3 0 0
БфЛЏСП 0.6 1.8 0.6 0.6
ЦНКтСП 0.4 1.2 0.6 0.6
ИїЮяжЪХЈЖШЗжБ№ЮЊc(CO2)=0.2mol/L, c(H2)=0.6mol/L, c(CH3OH)=0.3mol/L, c(H2O)=0.3 mol/LЃЛИљОнK=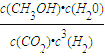 =0.3ЁС0.3/(0.2ЁС0.63)=2.08ЃЛе§ШЗД№АИЃК2.08ЁЃ
=0.3ЁС0.3/(0.2ЁС0.63)=2.08ЃЛе§ШЗД№АИЃК2.08ЁЃ
ЂлКуЮТКуШнЯТЃЌШнЦїAгыШнЦїBЮЊЕШаЇЦНКтЃЌЦНКтЪБЖдгІзщЗжЕФЮяжЪЕФСПЁЂХЈЖШЯрЕШЃЌЖўепЦ№ЪМЮяжЪЕФСПЕШгкИїЮяжЪЕФЛЏбЇМЦСПЪ§ЃЌЗХГіШШСПгыЮќЪеШШСПжЎКЭЕШгкЗДгІШШЪ§жЕЃЌдђЮќЪеЕФШШСП=ЗДгІШШ-ЗХГіЕФШШСП=49.0kJ-29.4kJ=19.6kJЃЛе§ШЗД№АИЃК19.6ЁЃ
ЂмвЊЪЙnЃЈCH3OHЃЉ/nЃЈCO2ЃЉдіДѓЃЌгІЪЙЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌ ЫљвдЃК
AЃЎвђе§ЗДгІЗХШШЃЌЩ§ИпЮТЖШЃЌЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌдђnЃЈCH3OHЃЉ/nЃЈCO2ЃЉМѕаЁЃЌЙЪAДэЮѓЃЛ
BЃЎГфШыЧтЦјЃЌЦНКтЯђе§ЗДгІЗНЗЈвЦЖЏЃЌnЃЈCH3OHЃЉ/nЃЈCO2ЃЉдіДѓЃЌЙЪBе§ШЗЃЛ
CЃЎНЋЫЎеєЦјДгЬхЯЕжаЗжРыЃЌЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌnЃЈCH3OHЃЉ/nЃЈCO2ЃЉдіДѓЃЌЙЪCе§ШЗЃЛ
DЃЎгУИќгааЇЕФДпЛЏМСЃЌЦНКтВЛвЦЖЏЃЌдђnЃЈCH3OHЃЉ/nЃЈCO2ЃЉВЛБфЃЌЙЪDДэЮѓЃЛ
е§ШЗбЁЯюЃКBCЁЃ


 аЁбЇЭЌВНШ§СЗКЫаФУмОэЯЕСаД№АИ
аЁбЇЭЌВНШ§СЗКЫаФУмОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНЋЯТСаЮяжЪЕФЫЎШмвКеєИЩВЂзЦЩеЃЌВЛФмЕУЕНИУЮяжЪЕФЪЧЃЈ ЃЉ
A.BaCl2B.NaHCO3C.Al(NO3)3D.Na2SO4
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЛЏКЯЮяY ФмгУгкИпадФмЙтбЇЪїжЌЕФКЯГЩЃЌПЩгЩЛЏКЯЮяXгы2 МзЛљБћЯЉѕЃТШдквЛЖЈЬѕМўЯТЗДгІжЦЕУЃК

ЯТСагаЙиЛЏКЯЮяXЁЂY ЕФЫЕЗЈе§ШЗЕФЪЧ
A. X ЗжзгжаЫљгадзгвЛЖЈдкЭЌвЛЦНУцЩЯ
B. YгыBr2ЕФМгГЩВњЮяЗжзгжаКЌгаЪжадЬМдзг
C. XЁЂYОљВЛФмЪЙЫсадKMnO4ШмвКЭЪЩЋ
D. XЁњYЕФЗДгІЮЊШЁДњЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПИљОнЯТСаЭМЪОЫљЕУГіЕФНсТлВЛе§ШЗЕФЪЧ

A. ЭММзЪЧCO(g)+H2O(g)![]() CO2(g)+H2(g)ЕФЦНКтГЃЪ§гыЗДгІЮТЖШЕФЙиЯЕЧњЯпЃЌЫЕУїИУЗДгІЕФІЄH<0
CO2(g)+H2(g)ЕФЦНКтГЃЪ§гыЗДгІЮТЖШЕФЙиЯЕЧњЯпЃЌЫЕУїИУЗДгІЕФІЄH<0
B. ЭМввЪЧЪвЮТЯТH2O2ДпЛЏЗжНтЗХГібѕЦјЕФЗДгІжаc(H2O2 )ЫцЗДгІЪБМфБфЛЏЕФЧњЯпЃЌЫЕУїЫцзХЗДгІЕФНјааH2O2ЗжНтЫйТЪж№НЅМѕаЁ
C. ЭМБћЪЧЪвЮТЯТгУ0.1000 molЁЄL1NaOHШмвКЕЮЖЈ20.00 mL 0.1000 molЁЄL1ФГвЛдЊЫсHXЕФЕЮЖЈЧњЯпЃЌЫЕУїHXЪЧвЛдЊЧПЫс
D. ЭМЖЁЪЧЪвЮТЯТгУNa2SO4Г§ШЅШмвКжаBa2+ДяЕНГСЕэШмНтЦНКтЪБЃЌШмвКжаc(Ba2+ )гыc(SO42)ЕФЙиЯЕЧњЯпЃЌЫЕУїШмвКжаc(SO42 )дНДѓc(Ba2+ )дНаЁ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТБэЮЊдЊЫижмЦкБэЕФвЛВПЗжЁЃ
ЬМ | ЕЊ | Y | |
X | Сђ | Z |
ЛиД№ЯТСаЮЪЬт
ЃЈ1ЃЉZдЊЫидкжмЦкБэжаЕФЮЛжУЮЊ__________ЁЃ
ЃЈ2ЃЉБэжадЊЫидзгАыОЖзюДѓЕФЪЧЃЈЬюдЊЫиЗћКХЃЉ__________ЁЃ
ЃЈ3ЃЉЯТСаЪТЪЕФмЫЕУїYдЊЫиЕФЗЧН№ЪєадБШSдЊЫиЕФЗЧН№ЪєадЧПЕФЪЧ__________ЃЛ
a.YЕЅжЪгыH2SШмвКЗДгІЃЌШмвКБфЛызЧ
b.дкбѕЛЏЛЙдЗДгІжаЃЌ1molYЕЅжЪБШ1molSЕУЕчзгЖр
c.YКЭSСНдЊЫиЕФМђЕЅЧтЛЏЮяЪмШШЗжНтЃЌЧАепЕФЗжНтЮТЖШИп
ЃЈ4ЃЉXгыZСНдЊЫиЕФЕЅжЪЗДгІЩњГЩ1molXЕФзюИпМлЛЏКЯЮяЃЌЛжИДжСЪвЮТЃЌЗХШШ687kJЃЌвбжЊИУЛЏКЯЮяЕФШлЁЂЗаЕуЗжБ№ЮЊ-69ЁцКЭ58ЁцЃЌаДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪН__________ЁЃ
ЃЈ5ЃЉЬМгыУОаЮГЩЕФ1molЛЏКЯЮяQгыЫЎЗДгІЃЌЩњГЩ2molMgЃЈOHЃЉ2КЭ1molЬўЃЌИУЬўЗжзгжаЬМЧтжЪСПБШЮЊ9:1ЃЌЬўЕФЕчзгЪНЮЊ__________ЁЃQгыЫЎЗДгІЕФЛЏбЇЗНГЬЪНЮЊ__________ЁЃ
ЃЈ6ЃЉЭгывЛЖЈХЈЖШЕФЯѕЫсКЭСђЫсЕФЛьКЯЫсЗДгІЃЌЩњГЩЕФбЮжЛгаСђЫсЭЃЌЭЌЪБЩњГЩЕФСНжжЦјЬхОљгЩБэжаСНжжЦјЬхзщГЩЃЌЦјЬхЕФЯрЖдЗжзгжЪСПЖМаЁгк50.ЮЊЗРжЙЮлШОЃЌНЋВњЩњЕФЦјЬхЭъШЋзЊЛЏЮЊзюИпМлКЌбѕЫсбЮЃЌЯћКФ1L2.2mol/LNaOHШмвККЭ1molO2ЃЌдђСНжжЦјЬхЕФЗжзгЪНМАЮяжЪЕФСПЗжБ№ЮЊ__________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЕтКЃДМЮЊЗЧРызгаЭдьгАМСЃЌЪЪгУгкгадьгАМСЗДгІЕФИпЮЃвђЫиЕФВЁШЫЁЃЯТУцЪЧвдЛЏКЯЮяAЮЊдСЯКЯГЩЕтКЃДМЕФТЗЯпЃК
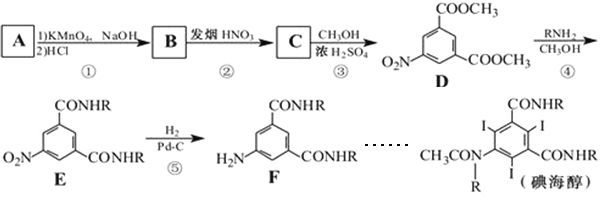
ЦфжаR-ЮЊ-CH2CH(OH)CH2OH
ЧыЛиД№вдЯТЮЪЬтЃК
ЃЈ1ЃЉЮяжЪDжаКЌгаЕФЙйФмЭХЮЊ________ЁЃ
ЃЈ2ЃЉAЪЧБНЕФЭЌЯЕЮяЃЌЯрЖдЗжзгСПЮЊ106ЃЌдђAЕФНсЙЙМђЪНЪЧ_______________ЁЃ
ЃЈ3ЃЉаДГіAЕФВрСДгыТШЦјЗЂЩњвЛТШШЁДњЕФЬѕМў_____________ЁЃ
ЃЈ4ЃЉЗДгІЂкЕФЛЏбЇЗНГЬЪН______________ЁЃ
ЃЈ5ЃЉЗДгІЂйЁњЂнжаЃЌЪєгкбѕЛЏЗДгІЕФЪЧ_____________ЃЈЬюађКХЃЉЁЃ
ЃЈ6ЃЉаДГіФмЭЌЪБТњзуЯТСаЬѕМўЕФDЕФСНжжЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃК_________ЁЃ
ЂёЃЎБНЛЗЩЯга3ИіШЁДњЛљЃЌБНЛЗЩЯЕФвЛТБДњЮяга2жжЃЛ
ЂђЃЎФмЗЂЩњвјОЕЗДгІЃЌЫЎНтВњЮяжЎвЛФмгыFeCl3ШмвКЗЂЩњЯдЩЋЗДгІЃЛ
ЂѓЃЎКЌга1ИіІСЃАБЛљЫсЕФНсЙЙЃЈР§ШчЃКCH3CH(NH2)COOHЪєгкІСЃАБЛљЫсЃЉ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖЬжмЦкдЊЫиRЁЂTЁЂQЁЂWдкдЊЫижмЦкБэжаЕФЯрЖдЮЛжУШчгвЯТЭМЫљЪОЃЌЦфжа T ЫљДІЕФжмЦкађЪ§гызхађЪ§ЯрЕШЁЃЯТСаХаЖЯВЛе§ШЗЕФЪЧ
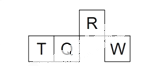
A. зюМђЕЅЦјЬЌЧтЛЏЮяЕФШШЮШЖЈадЃКR > Q
B. зюИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЕФЫсадЃКQ < W
C. дзгАыОЖЃКT > Q > R
D. КЌ T ЕФбЮШмвКвЛЖЈЯдЫсад
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПИпЬњЫсМи(K2FeO4)ЪЧвЛжжИпаЇЖрЙІФмЫЎДІРэМСЁЃЪЕбщЪвФЃФтЙЄвЕЩњВњK2FeO4ЕФСїГЬШчЯТЃК
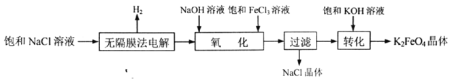
ЃЈ1ЃЉ K2FeO4МШФмЩБОњЯћЖОЃЌгжФмГСНЕЫЎжааќИЁЮяЃЌЦфдвђЪЧЃК_________________________________________ЁЃ
ЃЈ2ЃЉЮоИєФЄЗЈЕчНтБЅКЭNaClШмвКжЦБИNaClOзмЗДгІЕФРызгЗНГЬЪНЮЊЃК__________________ЁЃ
ЃЈ3ЃЉЁАбѕЛЏЁБЪБЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК_________________________________________ЁЃNa2FeO4ЁЂFeCl3ЁЂNaClOбѕЛЏадгЩЧПЕНШѕЕФЫГађЮЊ_________________________________ЁЃ
ЃЈ4ЃЉЁАЙ§ТЫЁБЫљЕУТЫвКжаЃЌГ§OHЃЭтЃЌвЛЖЈДцдкЕФвѕРызгЮЊ_____________________(ЬюРызгЗћКХ)ЁЃ
ЃЈ5ЃЉ ЪдДгГСЕэШмНтЦНКтЕФНЧЖШНтЪЭЁАзЊЛЏЁБЕУвдЫГРћЪЕЯжЕФдвђ___________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПбаОПNO2ЁЂNOЁЂCOЕШЮлШОЮяЕФДІРэЖдНЈЩшУРРіжаЙњОпгаживЊвтвхЁЃ
ЃЈ1ЃЉРћгУМзЭщДпЛЏЛЙдNOxЃК
CH4(g)+4NO2(g) ===4NO(g)+CO2(g)+2H2O(g) ІЄH1ЃНЃ574 kJЁЄmol-1
CH4(g)+4NO(g) ===2N2(g)+CO2(g)+2H2O(g) ІЄH2ЃНЃ1160 kJЁЄmol-1
ЂйМзЭщжБНгНЋNO2ЛЙдЮЊN2ЕФШШЛЏбЇЗНГЬЪНЮЊ________________________________ЁЃ
ЂкНЋCH4КЭNO2ГфШыУмБеШнЦїжаЗЂЩњЩЯЪіЗДгІЃЌИУЗДгІДяЕНЦНКтКѓЃЌЮЊСЫЬсИпЗДгІЫйТЪЕФЭЌЪБЬсИпNO2ЕФзЊЛЏТЪЃЌПЩВЩШЁЕФДыЪЉга__________(аДвЛЕуМДПЩ)ЁЃ
ЂлРћгУдЕчГиЗДгІПЩЪЕЯжNO2ЕФЮоКІЛЏЃЌзмЗДгІЮЊ6NO2+8NH3===7N2+12H2OЃЌЕчНтжЪШмвКЮЊNaOHШмвКЃЌЙЄзївЛЖЮЪБМфКѓЃЌИУЕчГие§МЋЧјИННќШмвКpH________(ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЃЌИКМЋЕФЕчМЋЗДгІЪНЮЊ___________________ЁЃ
ЃЈ2ЃЉЙтЦј(COCl2)дкЫмСЯЁЂжЦИяЁЂжЦвЉЕШЙЄвЕжагааэЖргУЭОЁЃЙЄвЕЩЯГЃРћгУЗЯЦјCO2ЭЈЙ§ЗДгІЃКC(s)+CO2(g)![]() 2CO(g) ІЄH>0ЃЌжЦШЁКЯГЩЙтЦјЕФдСЯЦјCOЁЃдкЬхЛ§ПЩБфЕФКубЙ(pзм)УмБеШнЦїжаГфШы1mol CO2 гызуСПЕФЬМЗЂЩњЩЯЪіЗДгІЃЌдкЦНКтЪБЬхЯЕжаЦјЬхЬхЛ§ЗжЪ§гыЮТЖШЕФЙиЯЕШчЭМЫљЪОЃК
2CO(g) ІЄH>0ЃЌжЦШЁКЯГЩЙтЦјЕФдСЯЦјCOЁЃдкЬхЛ§ПЩБфЕФКубЙ(pзм)УмБеШнЦїжаГфШы1mol CO2 гызуСПЕФЬМЗЂЩњЩЯЪіЗДгІЃЌдкЦНКтЪБЬхЯЕжаЦјЬхЬхЛ§ЗжЪ§гыЮТЖШЕФЙиЯЕШчЭМЫљЪОЃК
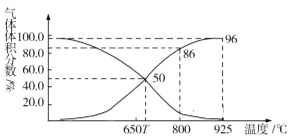
ЂйTЁцЪБЃЌдкШнЦїжаШєГфШыЯЁгаЦјЬхЃЌЦНКт______вЦЖЏ(ЬюЁАе§ЯђЁБЁАФцЯђЁБЛђЁАВЛЁБЃЌЯТЭЌ)ЃЛШєГфШыЕШЬхЛ§ЕФCO2КЭCOЃЌЦНКт________вЦЖЏЁЃ
ЂкCOЬхЛ§ЗжЪ§ЮЊ40%ЪБЃЌCO2ЕФзЊЛЏТЪЮЊ_______ЁЃ
ЂлвбжЊЃКЦјЬхЗжбЙ(pЗж)=ЦјЬхзмбЙЁСЬхЛ§ЗжЪ§ЁЃ800ЁцЪБгУЦНКтЗжбЙДњЬцЦНКтХЈЖШБэЪОЦНКтГЃЪ§Kp=______(гУКЌpзмЕФДњЪ§ЪНБэЪО)ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com