
�����ŷ�CO2����ɡ�����ЧӦ����Ϊ�˼���úȼ�նԻ�����ɵ���Ⱦ��ú�������Ǹ�Ч���������ú̿����Ҫ;����ú�ۺ����õ�һ��;����ͼ��ʾ��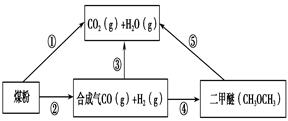
��1����֪��C(s) �� H2O(g) = CO(g)��H2(g) ��H1����131.3 kJ��mol��1
��C(s) �� 2H2O(g) = CO2(g) �� 2H2(g) ��H2����90 kJ��mol��1
��һ����̼��ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ�� ________________________��
��2������ͼԭ���װ�ÿ�����ɹ��̢ݵ�ת������װ��b�缫�ĵ缫��Ӧʽ��_______________________��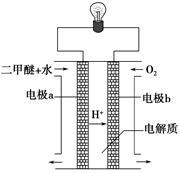
��3����ѹǿΪ0.1 MPa�����£��ݻ�ΪV L���ܱ�������a mol CO��2a mol H2�ڴ��������·�Ӧ���ɼ״���
CO(g)��2H2(g)  CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����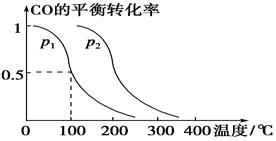
��p1________p2(�����������������)��
���������������������£���������������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ƽ��ת����________(���������С�����䡱)��
����p1�£�100 ��ʱ��CO(g)��2H2(g)  CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
��4����ͼ��ʾCO2��H2��Ӧ����CH3OH��H2O�Ĺ���������(��λΪkJ��mol��1)�ı仯��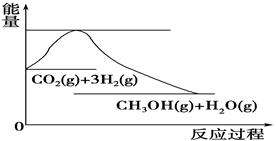
���ڸ÷�Ӧ������˵���У���ȷ����________(����)��
A����H��0����S��0 B����H��0����S��0
C����H��0����S��0 D����H��0����S��0
��5��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)  CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��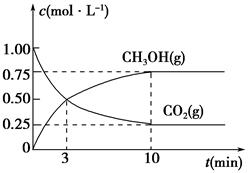
�ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)��________��
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����________(����)��
A�������¶� B����CH3OH(g)��ʱҺ���Ƴ�
C��ѡ���Ч���� D���ٳ���1 mol CO2��3 mol H2
��1�� CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.3 kJ��mol��1����2�� O2 +4e�� +2H2O = 4OH���� ��3���٣��� V 2 / a2���������� ��4��C�� ��5����0.075 mol/( L��min). ��BD
���������������1����-�ٿɵã�CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.3 kJ/mol.. ��2����ȼ�ϵ���У�ͨ��ȼ�ϵĵ缫��������ͨ�������ĵ缫��������a�缫�Ǹ�����b�缫��������b�缫�ĵ缫��Ӧʽ��O2 +4e�� +2H2O = 4OH������3�� ����ͼ���Կ��������¶���ͬʱ��ת����P2>P1������ƽ���ƶ�ԭ�����������������������¡�����ѹǿ����ѧƽ�������������С�ķ����ƶ�����������Ӧ�����ƶ�����ʱ��Ӧ���ת������ߡ�����P1<P2. ���������������������£���������������a mol CO��2a mol H2������������ϵ��ѹǿ����ʱ��ѧƽ��������Ӧ�����ƶ����ʴﵽ��ƽ��ʱ��CO��ƽ��ת����������p1�£�100 ��ʱ��CO(g)��2H2(g)  CH3OH(g)��Ӧ��ƽ�ⳣ��ΪK="C" (CH3OH)/ { C(CO)��C2(H2)} ="(" a/2V)��{(a/2V) ��(a/V)}2=" V" 2 / a2.��4��CO2(g)+3H2��g��
CH3OH(g)��Ӧ��ƽ�ⳣ��ΪK="C" (CH3OH)/ { C(CO)��C2(H2)} ="(" a/2V)��{(a/2V) ��(a/V)}2=" V" 2 / a2.��4��CO2(g)+3H2��g�� CH3OH(g)+CO(g).��ͼ�ɿ����÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���÷�Ӧ������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����ԡ�H<0����S<0��ѡ��Ϊ��C����5���ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=��1-0.25��mol/L��10min="0.075" mol/( L��min). ��A�����¶Ȼ�ѧƽ�������ȷ����ƶ������ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���������¶Ȼ�ѧƽ�����淴Ӧ�����ƶ���B����С�������Ũ�ȣ���ѧƽ��������Ӧ�����ƶ����ʽ�CH3OH(g)��ʱҺ���Ƴ���ʹƽ��������Ӧ�����ƶ���C������ �Ի�ѧƽ����Ӱ�졣D���ﵽƽ��ʱ���ٳ���1 mol CO2��3 mol H2����������ѹǿ����ѧƽ�������������С�ķ�������Ӧ�����ƶ���������ʹ��ѧƽ��������Ӧ�����ƶ��Ĵ�ʩ��BD��
CH3OH(g)+CO(g).��ͼ�ɿ����÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���÷�Ӧ������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����ԡ�H<0����S<0��ѡ��Ϊ��C����5���ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=��1-0.25��mol/L��10min="0.075" mol/( L��min). ��A�����¶Ȼ�ѧƽ�������ȷ����ƶ������ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���������¶Ȼ�ѧƽ�����淴Ӧ�����ƶ���B����С�������Ũ�ȣ���ѧƽ��������Ӧ�����ƶ����ʽ�CH3OH(g)��ʱҺ���Ƴ���ʹƽ��������Ӧ�����ƶ���C������ �Ի�ѧƽ����Ӱ�졣D���ﵽƽ��ʱ���ٳ���1 mol CO2��3 mol H2����������ѹǿ����ѧƽ�������������С�ķ�������Ӧ�����ƶ���������ʹ��ѧƽ��������Ӧ�����ƶ��Ĵ�ʩ��BD��
���㣺�����Ȼ�ѧ����ʽ����д����ѧƽ�ⳣ���ļ��㼰��������Ի�ѧƽ���Ӱ���֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ź㷺����;�������ڻ��ʡ����ᡢ�ϳ���ά�ȹ�ҵ������
��1���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɰ�����
�÷�Ӧ�ڹ̶�������ܱ������н��У��й�˵����ȷ����_____________���������ĸ����
A����Ӧ����ƽ��״̬ʱ��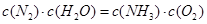 |
B����Ӧ�ﵽƽ���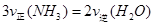 |
| C����ϵ����ѹǿ���䣬˵����Ӧ�Ѵ�ƽ�� |
| D�����������ܶȱ��ֲ��䣬˵����Ӧ�Ѵ�ƽ�� |
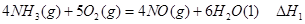 ��
��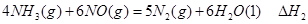 ��
��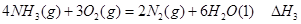 ��
��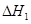 ��
��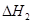 ��
��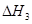 ����֮���ϵ�ı���ʽ��
����֮���ϵ�ı���ʽ��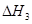 ��_________��
��_________��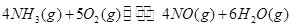
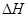 ��
��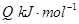


| ʱ��/Ũ�� |  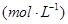 | 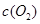 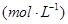 | 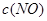 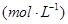 | 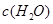  |
| ��ʼ | 4.0 | 5.5 | 0 | 0 |
| ��2min | 3.2 | a | 0.8 | 1.2 |
| ��4min | 2.0 | 3.0 | 2.0 | 3.0 |
| ��6min | 2.0 | 3.0 | 2.0 | 3.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����β���ﺬ�е�NO������������ȼ��ȼ�յĸ�����������������Ӧ���£�
N2(g)��O2(g)  2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��
2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��
��ش�
��1����֪��N2(g)+2O2(g)  2NO2(g) ��H1 2NO2(g)
2NO2(g) ��H1 2NO2(g)  O2+2NO(g) ��H2 ��H= ���ú���H1����H2�ı���ʽ��ʾ����
O2+2NO(g) ��H2 ��H= ���ú���H1����H2�ı���ʽ��ʾ����
��2��ij�¶��£���2 L���ܱ������г���N2��O2��1 mol��5���Ӻ�O2�����ʵ���Ϊ0.5 mol����NO�ķ�Ӧ���� ��
��3���ٶ��÷�Ӧ���ں��������½��У��������жϸ÷�Ӧ�Ѵﵽƽ�����________��
| A������1 mol N2ͬʱ����1 mol O2 |
| B����������ܶȲ��� |
| C���������ƽ����Է����������� |
| D��2v��(N2)��v��(NO) |
 2NO(g)�ġ�K-T������c(NO)-t��ͼ����ͼA������֪�÷�ӦΪ ��Ӧ������ȡ����ȡ�������ͼB��֪����a��Ӧ��������ȣ�b�ı������������ ��
2NO(g)�ġ�K-T������c(NO)-t��ͼ����ͼA������֪�÷�ӦΪ ��Ӧ������ȡ����ȡ�������ͼB��֪����a��Ӧ��������ȣ�b�ı������������ ��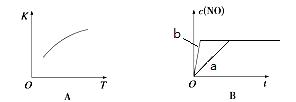
 2NO(g)________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��ƽ��ʱ��N2�ڻ�����������ٷ���Ϊ���٣����ڴ����д�����������̣��������2λ��Ч���֣�
2NO(g)________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��ƽ��ʱ��N2�ڻ�����������ٷ���Ϊ���٣����ڴ����д�����������̣��������2λ��Ч���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ڴ��������£�CO2��H2������ȡ�״����ù�ҵ�����е� ����ȡ�״����䷴ӦΪ��CO2+3H2
����ȡ�״����䷴ӦΪ��CO2+3H2 CH3OH+H2O ���³�ѹ����֪���з�Ӧ�������仯��ͼʾ��
CH3OH+H2O ���³�ѹ����֪���з�Ӧ�������仯��ͼʾ��
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ�� ��
�����⻯�ƣ�NaBH4�����л��ϳ��е���Ҫ��ԭ���������о����֣���NaBH4��H2O2Ϊԭ�ϣ�NaOH��Һ���������Һ��������Ƴ�ȫҺ����أ��乤��ԭ����ͼ��ʾ�������ع���ǰ����������Һ�������Ϊ1L���ش��������⣺
��1���缫bΪ ��������������������缫a�Ϸ�����Ӧ�ĵ缫��ӦʽΪ ��
��2����ع���ʱ��Na+�� �����a����b�����ƶ�������۲���0��0125molBO2������ʱ���Ҳ���ҺpH=
��3���øõ�ص��һ��Ũ�ȵ�CuSO4��Һ����ɫ��������һ��ʱ�䡣�Ͽ���·������Һ�м���0��1molCu(OH)2����Һ�ָ������֮ǰ״̬�����������ת�Ƶ�����ĿΪ_________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ѣ�CH3OCH3����һ����Ҫ�ľ�ϸ������Ʒ������Ϊ�Ƕ�ʮһ��������DZ����ȼ��[ ��֪��CH3OCH3(g)+3O2(g)��2CO2(g)+3H2O(1) ��H����1455kJ/mol ]��ͬʱ��Ҳ������Ϊ�������������ȴ�������ҵ���Ʊ������ѵ���Ҫ���������������Σ�
�ټ״�Һ����Ũ���������»�״������ڴ�������ֱ����ˮ�ƶ����ѣ�2CH3OH CH3OCH3��H2O
CH3OCH3��H2O
�ںϳ���CO��H2ֱ�Ӻϳɶ����ѣ�3H2(g)��3CO(g) CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol
����Ȼ����ˮ������Ӧ�Ʊ������ѡ���CH4��H2OΪԭ���Ʊ������Ѻͼ״���ҵ�������£�
��1��д��CO(g)��H2(g)��O2(g)��Ӧ����CO2(g)��H2O(1)���Ȼ�ѧ����ʽ���������һλС���� ��
��2���ٷ������ü״�Һ����Ũ��������ֱ����ˮ�ƶ����ѣ����ܲ��ʸߣ���������̭����Ҫԭ���� ��
��3���ڷ�Ӧ��2�У�һ�������·�����Ӧ3H2(g)��3CO(g) CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ��
CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ��
A�����¸�ѹ B���Ӵ��� C������COŨ�� D�������������
��4���ڷ�Ӧ��3�У���һ���¶Ⱥ�ѹǿ�����·����˷�Ӧ��3H2(g)��CO2(g)  CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ���
CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ���
A��P3��P2 T3��T2 B��P2��P4 T4��T2
C��P1��P3 T1��T3 D��P1��P4 T2��T3
��5����Ӧ��1�з�����Ӧ��CH4(g)��H2O(g) CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ�� ������¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ�� ��������䡱�����������С����
CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ�� ������¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ�� ��������䡱�����������С����
��6����ͼΪ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ����a�缫�ķ�ӦʽΪ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ܼ��š�������ȫ�����������ŷţ�����ʮ���ش�����̼�IJ�������ʵ������������ŵ���Ҫ;��֮һ����ѧ������ ��Һ���ܡ����������е�
��Һ���ܡ����������е� ��
��
��1��ʹ�ù��� ��Һ����
��Һ����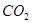 ����Ӧ�����ӷ���ʽΪ________��������3molNaOH����Һ��������22��4L
����Ӧ�����ӷ���ʽΪ________��������3molNaOH����Һ��������22��4L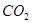 ���壨��״��������������Һ������̼Ԫ�ص������غ��ϵʽΪ__________��������Ũ�ȵĹ�ϵʽ��ʾ����
���壨��״��������������Һ������̼Ԫ�ص������غ��ϵʽΪ__________��������Ũ�ȵĹ�ϵʽ��ʾ����
��2������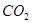 ��
��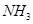 Ϊԭ�Ͽɺϳɻ�������[
Ϊԭ�Ͽɺϳɻ�������[ ]����֪��
]����֪��
 ��
��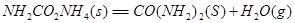
 ��
��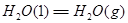
 ��
��
���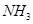 ��
��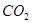 �ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ__________��
�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ__________��
��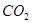 ͨ����Ӧ��ת��Ϊ
ͨ����Ӧ��ת��Ϊ ���ڴ���������CO��
���ڴ���������CO��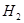 ��Ӧ���ɼ״���
��Ӧ���ɼ״��� ij�ݻ��ɱ���ܱ������г���10molCO��20mol
ij�ݻ��ɱ���ܱ������г���10molCO��20mol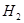 ��CO��ƽ��ת���ʣ�a�����¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��CO��ƽ��ת���ʣ�a�����¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
A����A���ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱ�������ݻ�ΪVL������¶��µ�ƽ�ⳣ��K=__________��ƽ��״̬B��ʱ�������ݻ� _______VL��������ڡ�����С�ڡ����ڡ���
_______VL��������ڡ�����С�ڡ����ڡ���
B����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��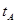 _______
_______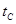 ���>������<����=����
���>������<����=����
C���ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ��________��д��һ�ּ��ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2012��ʼ������������������Ű���纪�������У�ȼú������β������ɿ�����Ⱦ��ԭ��֮һ��
��1������β����������Ҫԭ��Ϊ��2NO(g) + 2CO(g) 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
�ٸ÷�Ӧƽ�ⳣ������ʽ
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� ������ţ���
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
úȼ�ղ����������������������CH4����ԭNOX�������������������Ⱦ��
��֪���� CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)����H����867 kJ/mol
�� 2NO2(g) N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol
�� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ�� ��
��3������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��100mL1mol/Lʳ��ˮ,���һ��ʱ����ռ�����״���µ�����2.24L���������Һ������䣩.
�ټ���ȼ�ϵ�صĸ�����Ӧʽ�� ��
�ڵ�����Һ��pH= (��������������������Һ��Ӧ)
�������������������ڱ�״������ L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣���ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��C(s) +O2 (g)=CO2(g) ��H1<0 ��
;��II�����Ƴ�ˮú����C(s) +H2O(g)=CO(g)+H2(g) ��H2>0 ��
��ȼ��ˮú����2CO(g)+O2 (g)=2CO2(g) ��H3<0 ��
2H2(g)+O2 (g)=2H2O(g) ��H4<0 ��
��ش��������⣺
��1��;��I�ų������� ( ����ڡ������ڡ���С�ڡ�) ;��II�ų���������
��2����H1����H2����H3����H4����ѧ��ϵʽ�� ��
��3��12g̿���������в���ȫȼ������һ����̼���ų�110.35kJ���������Ȼ�ѧ����ʽΪ ��
��4��ú̿��Ϊȼ�ϲ���;��II���ŵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ����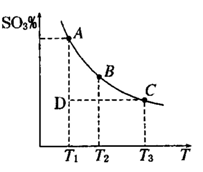
��1�����������У�SO2����������SO3�� 2SO2��g��+O2��g��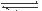 2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
��2SO2��g��+O2��g��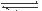 2SO3��g���ġ�H 0
2SO3��g���ġ�H 0
���>����<���������ں��¡���ѹ������������ƽ����ϵ��ͨ�뺤����ƽ�� �ƶ�����������ҡ�������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1 K2����Ӧ���е�״̬Dʱ�� 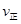
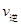 ���>����<����=����
���>����<����=����
��2�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũ ҵ������������������Ҫ���ã�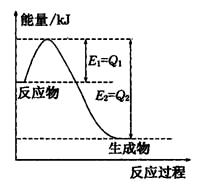
����ͼ��һ�����¶Ⱥ�ѹǿ��N2��H2��Ӧ����lmolNH3�����������仯ʾ��ͼ����д����ҵ�ϳɰ����Ȼ�ѧ����ʽ��
����H����ֵ�ú���ĸQ1��Q2�Ĵ���ʽ��ʾ��
�ڰ�������ˮ�õ���ˮ����25���£���a mol��L-1�İ�ˮ��b mol��L-1������������ϣ���Ӧ����Һ�����ԣ���c��NH4+�� c��Cl-�����>������<����=�������ú�a��b�Ĵ���ʽ��ʾ���û����Һ�а�ˮ�ĵ���ƽ�ⳣ�� .
��3����ˮ�к��д�����Ԫ�أ�����Ԫ�����ȣ���Ԫ����⣬���ں�ˮ�о��Ի���̬���ڣ���25���£���0��1L0.002mol��L-l��NaCl��Һ����μ���������0��1L0.002mol��L-l��������Һ���а�ɫ�������ɣ��ӳ����ܽ�ƽ��ĽǶȽ��Ͳ���������ԭ���� ����Ӧ�����Һ�м�������0��1L0.002mol��L-1��NaI��Һ�������������� �������������ԭ���ǣ������ӷ���ʽ��ʾ�� ��
����֪��25��ʱKSP��AgCl��=1.6��l0-10 KSP��AgI��=1.5��l0-16��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com