
���� ��1��ͨ��״���£�0.25molCO����ȫȼ������CO2�����ų�70.75kJ����������2mol��һ����̼�ų�����Ϊ70.75kJ��$\frac{2}{0.25}$=566kJ/mol���ɴ���д�Ȼ�ѧ����ʽ��
��2�����趼ȼ��1mol�����壬ú����CO��g�������ʵ���Ϊ0.8mol��H2��g������������Ϊ0.2mol���ų�������Ϊ��$\frac{0.8}{0.25}$��70.75kJ+0.2mol��286.0kJ=226.4kJ+57.2kJ=283.6kJ����1mol����ȼ���ų�����Ϊ890.0kJ��Ȼ��Ƚ����ߵĴ�С�ó����ۣ�
��3�����������غ㶨�ɺͻ��Ϸ�Ӧ�Ķ�����ص�����������Ҫ�����÷�Ӧǰ��ԭ�ӵ�����䣬����Ŀ�ر��ֲ���������
��4����Ϊ�ϳ��������ʺ�ˮ������һ����̼�е���ȫ��ת��Ϊˮ�е������������غ㣬����ˮ�����ʵ���Ϊxmol������ˮ����ɣ����ˮ����������ʵ���Ϊ2xmol����������̼�����ʵ���Ϊxmol��������ʵ���Ϊ��2y-2x�����ɴ˵ó���ѧʽ��
��5��������һ����̼����������Ӧ���ڼ�������������̼���Σ����Ե缫��ӦʽΪ��CO-2e-+40H-=CO32-+2H2O���ɵ缫��Ӧʽ��֪ת��2mol�ĵ�������1mol��һ����̼���ɴ˷������
��� �⣺��1��ͨ��״���£�0.25molCO����ȫȼ������CO2�����ų�70.75kJ����������2mol��һ����̼�ų�����Ϊ70.75kJ��$\frac{2}{0.25}$=566kJ/mol�������Ȼ�ѧ����ʽΪ��2CO��g��+O2��g��=2CO2��g����H=-566.0 kJ/mol���ʴ�Ϊ��2CO��g��+O2��g��=2CO2��g����H=-566.0 kJ/mol��
��2�����趼ȼ��1mol�����壬ú����CO��g�������ʵ���Ϊ0.8mol��H2��g������������Ϊ0.2mol���ų�������Ϊ��$\frac{0.8}{0.25}$��70.75kJ+0.2mol��286.0kJ=226.4kJ+57.2kJ=283.6kJ����1mol����ȼ���ų�����Ϊ890.0kJ����Ϊ��890.0kJ��283.6kJ��������Ȼ���ų������࣬�ʴ�Ϊ����Ȼ����
��3����Ӧ���е�ԭ�������ʴﵽ100%��Ҳ����˵��Ӧ����һ���ǻ��Ϸ�Ӧ���ҷ�Ӧ������еĸ�ԭ����Ŀ�Ȳ��䣬CO��H2��һ�������°��ղ�ͬ�ı�����Ӧ����ֻҪ��ѧʽ�ܻ�Ϊ��ʽ��CO��n��H2��m�������ԣ�ֻ����һ��̼ԭ�ӵ��л������ԭ����Ӧ��С��4��ż��������n=1��m=2��
n=1��m=4����Ϊ����ȩ���״����ʴ�Ϊ����ȩ���״���
��4����Ϊ�ϳ��������ʺ�ˮ������һ����̼�е���ȫ��ת��Ϊˮ�е������������غ㣬����ˮ�����ʵ���Ϊxmol������ˮ����ɣ����ˮ����������ʵ���Ϊ2xmol����������̼�����ʵ���Ϊxmol��������ʵ���Ϊ��2y-2x�����ɴ˵ó����Ļ�ѧʽΪ��CxH��2y-2x�����ʴ�Ϊ��CxH��2y-2x����
��5��������һ����̼����������Ӧ���ڼ�������������̼���Σ����Ե缫��ӦʽΪ��CO-2e-+40H-=CO32-+2H2O���ɵ缫��Ӧʽ��֪ת��2mol�ĵ�������1mol��һ����̼�����Ե���·��ͨ��0.2mol����ʱ�����ĵ�COΪ0��lmol���ʴ�Ϊ��CO-2e-+40H-=CO32-+2H2O��0��l��
���� ���⿼�����Ȼ�ѧ����ʽ����д��ԭ��ع���ԭ������ɫ��ѧ����Ŀ�ѶȽϴ����������ϴ�֪ʶ��϶࣬��ֿ�����ѧ������ѧ֪ʶ�������������������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ˮ | B�� | �á�84����Һ������ | ||
| C�� | ������ᴿ | D�� | ��С�մ�����θ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | C�� | ̫���� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������ | ��ѧ������ | ���������� |
| HCl | ���ۼ� | ���ۼ������� |
| NaCl | ���Ӽ� | ���ӻ����� |
| NaOH | ���Ӽ����ۼ� | ���ӻ����� |
| NH4Cl | ���Ӽ����ۼ� | ���ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ���ȴ���������ԭ�Ӽ��ִ��������ڷ��Ӽ� | |
| B�� | ����ԭ�Ӽ������ýл�ѧ�� | |
| C�� | ��ѧ��ͨ��ָ�������ڵ���������ԭ��֮���ǿ�ҵ������ | |
| D�� | ��ѧ����ʵ���Ǿ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ�������ͭƬ�ϣ�Cu+2H+=Cu2++H2�� | |
| B�� | ����������ϡ�����ϣ�SO42-+H++OH-+Ba2+=H2O+BaSO4�� | |
| C�� | ��ϡ����ϴȥ���⣺Fe2O3+6H+=2 Fe3++3H2O | |
| D�� | ʯ������Na2CO3��Һ��ϣ�Ca2++CO32-=CaCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
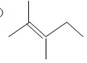
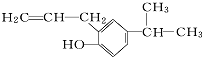 �ķ���ʽΪC12H16O��
�ķ���ʽΪC12H16O�� ������������11��̼ԭ�Ӵ���ͬһƽ���ϣ�
������������11��̼ԭ�Ӵ���ͬһƽ���ϣ�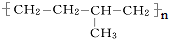 �ĵ���Ϊ
�ĵ���Ϊ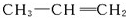 ��
�� ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com