








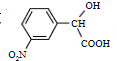
 ������ͬ���칹�����������������������ڱ��Ķ�λ��Ԫȡ����ں����������ǻ����Ȼ����۷������������ͬ���칹��Ϊ��
������ͬ���칹�����������������������ڱ��Ķ�λ��Ԫȡ����ں����������ǻ����Ȼ����۷������������ͬ���칹��Ϊ�� ��
�� ��
�� ��
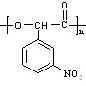
�� ������ͬ���칹�����������������������ڱ��Ķ�λ��Ԫȡ����ں����������ǻ����Ȼ����۷������������ͬ���칹��Ϊ
������ͬ���칹�����������������������ڱ��Ķ�λ��Ԫȡ����ں����������ǻ����Ȼ����۷������������ͬ���칹��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� �����Է�Ӧ����ʽΪ
�����Է�Ӧ����ʽΪ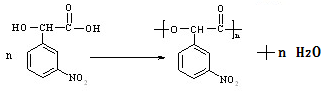 ��
�� ��
�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


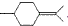 ���������մ��Ľṹ��ʽΪ
���������մ��Ľṹ��ʽΪ

 �ĺϳ�·������ͼ�������Լ����ã��ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�������Լ����ã��ϳ�·������ͼʾ�����£� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
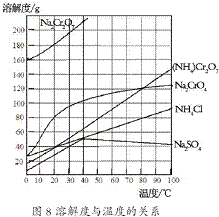 ��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�
��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�2- 3 |
2- 6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
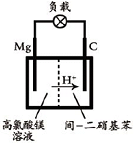 ��2012?����һģ��þ����Ͻ�㷺Ӧ���ں��պ��졢��ͨ����ص���ҵ������þ���Ʊ�������Ҫ�У�
��2012?����һģ��þ����Ͻ�㷺Ӧ���ں��պ��졢��ͨ����ص���ҵ������þ���Ʊ�������Ҫ�У�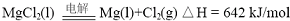
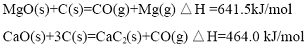
| n��CaC2��/N��MgO�� | ��ԭ�¶�/�� | ����ʱ��/h | ��ԭ��/% |
| 1.1 | 1110 | 2.0 | 62 |
| 1.1 | 1150 | 2.0 | 80 |
| 1.1 | 1150 | 2.5 | 85 |
| 1.2 | 1000 | 2.0 | 33 |
| 1.2 | 1150 | 2.0 | 84 |
| 1.2 | 1150 | 2.5 | 88 |
| 1.3 | 1150 | 2.0 | 86 |
| 1.3 | 1150 | 2.0 | 88 |
| 1100-1250�� |
| ��� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�




�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com