
��ˮFeCl3���غ�ɫ�����׳��⣬100������ʱ��������ҵ�ϳ������л��ϳɴ�����ʵ���ҿ�������װ��(�г�������ȥ)�Ʊ����ռ���ˮFeCl3��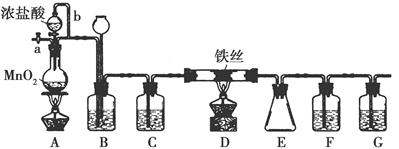
(1)װ��A�з�Ӧ�����ӷ���ʽΪ______________________��
(2)װ��F�����ӵ��Լ�Ϊ________��
(3)����b������Ϊ________��װ��B������Ϊ___________��
(4)ʵ��ʱӦ�ȵ�ȼA���ľƾ��ƣ���Ӧһ������ٵ�ȼD���ľƾ��ƣ�ԭ��Ϊ____________________________________��
(5)��Ӧ������жװ��ǰ��������еIJ�����__________��
(6)Ϊ�������ò�Ʒ���Ƿ���FeCl2���ɽ�������ʵ�飺ȡE���ռ��IJ�����������ˮ�ܽ⣬��������Һ�м���һ���Լ������Լ�Ϊ________(�����)��
��Fe�� ��KSCN��Һ ������KMnO4��Һ ��NaOH��Һ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʡ��2013��12��1����ʱ�𣬳�����������Ϊ�������������Զ���������ŷ����˴��ĸ��ơ���֪SO2������Fe( NO3)3��Һ���գ� 0��1mol/L��Fe(NO3)3��Һ��pH��2��ijѧϰС��ݴ�չ���������̽����
��̽��I��ͭ��Ũ����ķ�Ӧ̽����
(l)ȡ12��8gͭƬ��������ƿ�У�ͨN2һ��ʱ����ټ���20 mL 18 mol?L-1��Ũ���ᣬ���ȡ�װ��A���а������������������ɣ�װ��B�в�����ɫ��������ַ�Ӧ��,��ƿ������ͭƬʣ�ࡣ
�ٸ�С��ͬѧ��Ϊ��ƿ�г���ͭƬʣ���Ӧ�н϶������ʣ�࣬��ԭ���ǣ� ___________________ ��
�ڸ�С��ͬѧ��ͨ���ⶨ�����������������������ʵ���������˶���ʵ�鷽�������з��������е���______ ��
A�������������建��ͨ��Ԥ�ȳ�����ʢ�м�ʯ�ҵĸ����,������Ӧ���ٴγ���
B�������������建��ͨ�����������ữ�ĸ��������Һ���ټ���������BaCl2��Һ���������ó���������
C�����ű���NaHSO3��Һ�ķ����ⶨ�������������(����ɱ�״����
��̽��II��װ��B�в���������ԭ��̽����
��2������Ũ����֮ǰ��ͨN2һ��ʱ�䣬��Ŀ����____ ��
��3���������ۣ���С���װ��B�в���������ԭ��������в���(�����Ǹ����صĵ��ӣ���
����1: װ��A�еİ�������B���뷴Ӧ
����2��SO2��Fe3+����ΪSO42-
����3�� ��
��4����ͬѧ��ΪֻҪ��װ��A��B������ϴ��ƿC���Ϳ����ų�װ��A�а���Ӱ�죬��C��ʢ�ŵ��Լ��� ��
��5����ͬѧȡ������װ��B����Һ�����뼸�����Ը�����أ������Ϻ�ɫ��ȥ���ݴ���Ϊ����2���������Ƿ�ͬ������ۣ���˵�����ɣ� ��
��˼���뽻����
��6��ʵ���������ʹ��ƿ��ͭƬ�����ܽ⣬���з���(��Ҫʱ�ɼ��ȣ����е��� ��
A�����ɼУ�ͨ��O2 B���ɷ�Һ©������H2O2��Һ
C���ɷ�Һ©������NaNO3��Һ D���ɷ�Һ©������Na2SO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�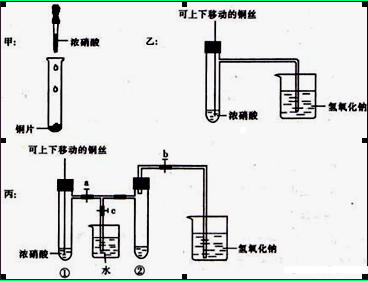
��1���ס��ҡ�������װ���й�ͬ�����Ļ�ѧ����ʽ�� ��
��2���ͼ�װ����ȣ���װ�õ��ŵ��ǿ��Կ��Ʒ�Ӧ�Ŀ�ʼ��ֹͣ�������� ��
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��������������Թܺ�ͭ˿��������Һ���롣��ʹ�ձ��е� ˮ���ˢ��Թܣ�Ӧ��β���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ü�������������ȡ����Ӧ��ȡ����Ʒ����������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС����ʵ������ģ���������̣�����Ƶ�ģ��װ�����£�
�������Ҫ��ش�
��1��Bװ�������ֹ��ܣ��ٿ��������ٶȣ��ھ��Ȼ�����壻��______________��
��2����V��Cl2��/V��CH4����x��������������������Ȼ��⣬��xֵӦ________��
��3��Dװ�õ�ʯ���о��Ȼ���KI��ĩ����������____________��
��4��Eװ�õ�������________������ţ���
| A���ռ����� | B���������� |
| C����ֹ���� | D�������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ����С��ͨ��ʵ���о�NO2�����ʡ�
��֪:2NO2+2NaOH NaNO3+NaNO2+H2O
NaNO3+NaNO2+H2O
����1:������ͼ��ʾװ��̽��NO2�ܷ�NH3��ԭ(K1��K2Ϊֹˮ��,�г̶ֹ�װ����ȥ)��
(1)Eװ������ȡNO2��Ӧ�Ļ�ѧ����ʽ�� ����
(2)��NO2�ܹ���NH3��ԭ,Ԥ�ڹ۲쵽Cװ���е��������� ��
(3)ʵ�������,δ�ܹ۲쵽Cװ���е�Ԥ������С��ͬѧ�ӷ�Ӧԭ���ĽǶȷ�����ԭ��,��Ϊ������:
��NH3��ԭ�Խ���,���ܽ�NO2��ԭ;
���ڴ�������,NO2��ת���ʼ���;
���� ��
(4)��ʵ��װ�ô���һ�����Ե�ȱ���� ��
����2:̽��NO2�ܷ���Na2O2����������ԭ��Ӧ��
(5)ʵ��ǰ,��С��ͬѧ������ּ��衣
����1:���߲���Ӧ;
����2:NO2�ܱ�Na2O2����;
����3:���������������������� ��
(6)Ϊ����֤����2,��С��ͬѧѡ������1�е�B��D��Eװ��,��B�е�ҩƷ����ΪNa2O2,��ѡFװ��(��ͼ��ʾ),������װ,����ʵ�顣
��װ�õĺ�������˳������ ��
��ʵ�������,Bװ���е���ɫ��ĩ��ɰ�ɫ��������,�ð�ɫ����Ϊ������,���������������ɡ��Ʋ�Bװ���з�Ӧ�Ļ�ѧ����ʽΪ����������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������ͭ��ȡ����ͭ������������ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�õ�����ͭ���塣
(1)Ϊ�˽�Լԭ�ϣ�����������������ʵ���֮��Ϊ________ ��Ϊ�����ո÷�Ӧ�в�����β������ѡ������װ����________(�����)��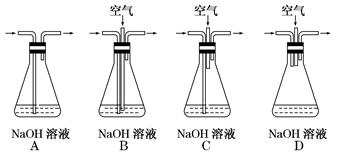
(2)Ϊ������ɫ��ѧ��Ҫ��ij�о���ѧϰС�����������ơ�
����1���Կ���Ϊ����������ͭ��������N�з������գ�ʹͭ�������ַ�Ӧ��������ͭ����ʹ����ͭ��ϡ���ᷴӦ��
����2��������������ֱ��ͨ�뵽ͭ����ϩ����Ļ�����У������ڳ����¼�������Ӧ����ӦҺ�м�����FeSO4����ͨ���������������������Ӧ����������ͭ����Ӧ��ȫ�������ʼ���pH����Ԫ��ȫ��ת��ΪFe(OH)3����(һ�㵱�����ӵ�Ũ���½���10��5mol��L��1ʱ������Ϊ������ȫ)��Ȼ����ˡ�Ũ�����ᾧ��
(��֪�������£�Ksp[Cu(OH)2]��10��22��Ksp[Fe(OH)3]��10��38)
��ش��������⣺
�ٷ���1������N��������________��
�ڷ���2��Ϊ��ʹ��Ԫ��ȫ��������Ӧ����pH����Ϊ________��
�۷���2�м����ʿ�ѡ��________(�����)��
| A��CaO | B��NaOH | C��CuCO3 | D��Cu2(OH)2CO3 E��Fe2(SO4)3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(1)ij��ѧ��ȤС���ͬѧ����Cl2��NH3���Ʊ������ʼ����ʵ������̺Ͳ���װ�����£�
��������A��Gװ�����һ����ʵ����֤Cl2��Fe3����I2��������ǿ��ΪCl2>Fe3��>I2(ʵ���в��ϵ�С����Gװ���е��Թ�)����д��A�з�����Ӧ�����ӷ���ʽ��______________________________________________��
��д���Լ�MΪ________��Һ��֤��������ΪCl2>Fe3��>I2��ʵ��������________________________________________________________________________��
����֪3Cl2��2NH3=6HCl��N2����D����ƿ�г�������ɫ����ر�a��c��b��D�е�����Ϊ����ɫ������ʧ���������̣���Ӧһ��ʱ��ر�b��c���۲쵽������Ϊ________________________________________________________________________��
(2)ij��ˮ�к���һ������Na����SO32�������ܺ���CO32����ij�о�С�����ⶨ����SO32����Ũ�ȣ��������ʵ�鷽����
�ٴ������Լ���ѡ���Լ�XΪ________(�����)��
A��0.1 mol/L KMnO4(H2SO4�ữ)��ҺB��0.5 mol/L NaOH��Һ
C��������ˮ D��KI��Һ
�ڼ����Լ�X����SO42�������ӷ���ʽΪ_______________________________________
��֤���÷�ˮ���Ƿ���CO32����ʵ�鷽��Ϊ_________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ��,CΪ��������������백����Ӧ��װ�á�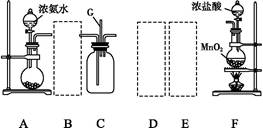
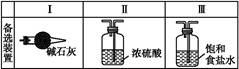
(1)װ��F�з�����Ӧ�����ӷ���ʽΪ�� ��
(2)װ��A�е���ƿ�ڹ����ѡ����������(������ѡ��Ĵ���)��
A.��ʯ�� B.��ʯ�� C.�������� D.���������� E.�ռ�
(3)���߿���Ӧ���ӱ�Ҫ�ij���װ��,�����ͼ�ı�ѡװ����ѡ��,��������������пո�:B��������,D��������,E��������(������)��
(4)�����Ͱ����ڳ��������ͻᷴӦ�����Ȼ�狀͵���,װ��C�ڳ���Ũ��İ��̲��������ڱ�����,�����ʵ�鷽�������ù�������Ȼ��: ��
(5)��װ��C�ij����ܿڴ��ݳ���β�����ܺ�����Ⱦ����������,��δ���?
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ����ҵ���ó�ʪ��KClO3�Ͳ��ᣨH2C2O4����60ʱ��Ӧ�Ƶá�ijѧ����������ͼ��ʾ��װ��ģ����ȡ���ռ�ClO2��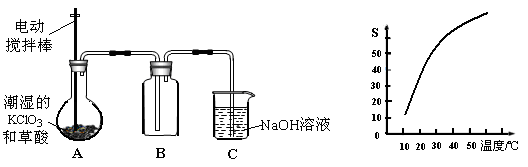
��1��A�з�Ӧ������K2CO3��ClO2��CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ��� ��BҲ���������¶ȿ���װ�ã�Ӧ���� (ѡ���ˮԡ������ˮԡ��)װ�á�
��3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ�����¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ��벹���NaClO2��Һ���Ƶ�NaClO2�IJ������裺
�� ���� ����ϴ�ӣ��ܸ��
��4��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ����������ǰ���NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2+�����ʵ�����ͬ���ӵ����غ�ĽǶȽ�����ԭ���� ��
ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00 mL��ϡ�ͳ�100.00 mL��������ȡV1 mL�������뵽��ƿ�У�
����2������������pH��2.0������������KI���壬����Ƭ�̣�
����3���������ָʾ������c mol��L��1 Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2 mL������֪2 Na2S2O3 + I2 ��Na2S4O6 + 2NaI��
��5���жϵζ��յ������ ��ԭClO2��Һ��Ũ��Ϊ g / L���ò����е���ĸ����ʽ��ʾ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com