
ѕ¬Ѕ– « µ—й£®1£©°Ђ£®5£©Ћщњіµљµƒѕ÷ѕу£ђ ‘їЎірќ ћв£Ї
£®1£©Љ”≈®ЅтЋб”ЏЊІћеA ±£ђЈ≈≥ц∆шћеB£ђB”цµљC ±ЊЌ≤ъ…ъ∞„—ћ.
£®2£©‘ЏBµƒЋЃ»№“Ї÷–£ђљр фMњ…“‘»№љв£ђґшљр фN≤ї»№љв.
£®3£©‘Џ≈®ѕхЋб÷–£ђљр фM≤ї»№љв£ђґшљр фNњ…“‘»№љв.
£®4£©…ѕ цљр фјл„”M3+ЇЌN2+µƒЋЃ»№“ЇґЉЊя”–—’…Ђ£ђ’в–©»№“Ї≥дЈ÷ќь ’C“‘Їу£ђMµƒїѓЇѕќп±д≥…≥Ѕµн£ђNµƒїѓЇѕќп‘т»№љв.
£®5£©‘ЏAµƒ±•ЇЌ»№“Ї÷–≥дЈ÷ќь ’CЇу£ђ‘ўѕт∆д÷–Ќ®»лґю—хїѓћЉ ±£ђDЊЌ≥Ѕµн≥цјі.
ќ ћв£Ї£®a£©–і≥цїѓЇѕќпA°ҐB°ҐCµƒїѓ—І љ.
£®b£©‘Џ µ—й£®2£©÷–£ђљр фM»№љв£ђљр фN≤ї»№љв£ђ « ≤√і‘≠“т?
£®c£©‘Џ µ—й£®3£©÷–£ђ”…”Џљр фNµƒ»№љв£ђЈ≈≥цµƒ « ≤√і∆шће?
£®d£©–і≥ц µ—й£®4£©÷–Ћщ…ъ≥…µƒM°ҐNµƒїѓЇѕќпµƒїѓ—І љЇЌ—’…Ђ.
£®e£©–і≥ц µ—й£®5£©÷–…ъ≥…Dµƒїѓ—ІЈљ≥ћ љ.
£®a£©A «NaCl£ђB «HCl£ђC «NH3£ї £®b£©“тќ™N±»«вµƒјл„”їѓ«гѕт–°£ђM±»«вµƒјл„”їѓ«гѕтіу£ї £®c£©ґю—хїѓµ™£ї £®d£©M£Ї £®e£©
|
B”цµљC ±ЊЌ≤ъ…ъ∞„—ћ‘т÷™ґю’яќ™HClЇЌNH3£ђB «”…Љ”≈®ЅтЋбµ√µљ£ђЋщ“‘B «HCl£ђCќ™NH3£ђ‘тAќ™NaCl£ђќ™ µ—й “÷∆±ЄHClµƒЈљЈ®£ї£®2£©£®3£©÷™Mњ…“‘±ї≈®ЅтЋбґџїѓ£ђќ™ћъїт¬Ѕ£ђMњ…“‘–ќ≥…’э»эЉџµƒјл„”£ђ«“∆д»№“ЇЊя”–—’…Ђ£ђЋщ“‘Mќ™Fe£ђ”…£®2£©£®3£©£®4£©њ…“‘Ќ∆≤вљр фNќ™Cu.
|



| ƒкЉґ | Єя÷–њќ≥ћ | ƒкЉґ | ≥х÷–њќ≥ћ |
| Єя“ї | Єя“ї√вЈ—њќ≥ћЌ∆Љц£° | ≥х“ї | ≥х“ї√вЈ—њќ≥ћЌ∆Љц£° |
| Єяґю | Єяґю√вЈ—њќ≥ћЌ∆Љц£° | ≥хґю | ≥хґю√вЈ—њќ≥ћЌ∆Љц£° |
| Єя»э | Єя»э√вЈ—њќ≥ћЌ∆Љц£° | ≥х»э | ≥х»э√вЈ—њќ≥ћЌ∆Љц£° |
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї ћв–Ќ£Ї
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Їќпјнљћ—– “ ћв–Ќ£Ї022
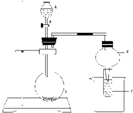
£®1£©Љ”≈®ЅтЋб”ЏЊІћеA ±£ђЈ≈≥ц∆шћеB£ђB”цµљC ±ЊЌ≤ъ…ъ∞„—ћ.
£®2£©‘ЏBµƒЋЃ»№“Ї÷–£ђљр фMњ…“‘»№љв£ђґшљр фN≤ї»№љв.
£®3£©‘Џ≈®ѕхЋб÷–£ђљр фM≤ї»№љв£ђґшљр фNњ…“‘»№љв.
£®4£©…ѕ цљр фјл„”M3+ЇЌN2+µƒЋЃ»№“ЇґЉЊя”–—’…Ђ£ђ’в–©»№“Ї≥дЈ÷ќь ’C“‘Їу£ђMµƒїѓЇѕќп±д≥…≥Ѕµн£ђNµƒїѓЇѕќп‘т»№љв.
£®5£©‘ЏAµƒ±•ЇЌ»№“Ї÷–≥дЈ÷ќь ’CЇу£ђ‘ўѕт∆д÷–Ќ®»лґю—хїѓћЉ ±£ђDЊЌ≥Ѕµн≥цјі.
ќ ћв£Ї£®a£©–і≥цїѓЇѕќпA°ҐB°ҐCµƒїѓ—І љ.
£®b£©‘Џ µ—й£®2£©÷–£ђљр фM»№љв£ђљр фN≤ї»№љв£ђ « ≤√і‘≠“т?
£®c£©‘Џ µ—й£®3£©÷–£ђ”…”Џљр фNµƒ»№љв£ђЈ≈≥цµƒ « ≤√і∆шће?
£®d£©–і≥ц µ—й£®4£©÷–Ћщ…ъ≥…µƒM°ҐNµƒїѓЇѕќпµƒїѓ—І љЇЌ—’…Ђ.
£®e£©–і≥ц µ—й£®5£©÷–…ъ≥…Dµƒїѓ—ІЈљ≥ћ љ.
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї2011-2012—Іƒкљ≠ќч…ѕ»ƒ÷–—ІЄя“їЅгµг°Ґ µ—й∞аѕ¬∆Џƒ©їѓ—І ‘Њн£®ішљвќц£© ћв–Ќ£Ї µ—йћв
£®14Ј÷£©ѕ¬√ж «Љ„°Ґ““°Ґ±ы»эќїЌђ—І÷∆»°““Ћб““х•µƒєэ≥ћ£ђ«лƒг≤ќ”л≤Ґ–≠÷ъЋы√«Ќк≥…ѕаєЎ µ—й»ќќс°£
[ µ—йƒњµƒ]÷∆»°““Ћб““х•
[ µ—й‘≠јн]Љ„°Ґ““°Ґ±ы»эќїЌђ—ІЊщ≤…»°““іЉ(CH3CH218OH)°Ґ““Ћб”л≈®ЅтЋбїмЇѕє≤»»µƒЈљЈ®÷∆»°““Ћб““х•£ђ∆д÷–≈®ЅтЋб≥эЅЋ„чќьЋЃЉЅµƒ„ч”√Ќвїє”–
[„∞÷√…иЉ∆]Љ„°Ґ““°Ґ±ы»эќїЌђ—ІЈ÷±р…иЉ∆ЅЋѕ¬Ѕ–»эћ„ µ—й„∞÷√£Ї
«лі”Љ„°Ґ““ЅљќїЌђ—І…иЉ∆µƒ„∞÷√÷–—°‘с“ї÷÷„чќ™ µ—й “÷∆»°““Ћб““х•µƒ„∞÷√£ђќ“—°‘сµƒ„∞÷√ « (—°ћо °∞Љ„°±їт °∞““°±)°£±ыЌђ—ІљЂЉ„„∞÷√÷–µƒ≤£ЅІє№Єƒ≥…ЅЋ«т–ќЄ…‘пє№≥э∆рјдƒэ„ч”√Ќв£ђЋьµƒЅн“ї÷Ў“™„ч”√ « 
[ µ—й≤љ÷и](1)∞і—°‘сµƒ„∞÷√„й„∞“«∆ч£ђ‘Џ ‘є№÷–ѕ»Љ”»л3mLCH3CH218OH£ђ2mL±щі„Ћб£ђ≤Ґ‘Џ“°ґѓѕ¬їЇїЇЉ”»л2mL≈®ЅтЋб≥дЈ÷“°‘»£ђ (2)љЂ ‘є№єћґ®‘ЏћъЉ№…ѕ£ї(3)‘Џ ‘є№ҐЏ÷–Љ”»Ћ Ѕњµƒ±•ЇЌNa2CO3»№“Ї£ї(4)”√Њ∆ЊЂµ∆ґ‘ ‘є№ҐўЉ”»»£ї(5)µ±єџ≤мµљ ‘є№ҐЏ÷–”–√чѕ‘ѕ÷ѕу ±Ќ£÷є µ—й°£
[ќ ћвћ÷¬џ]a£ЃЄщЊЁ ‘є№ҐЏ÷–єџ≤мµљµƒѕ÷ѕу£ђњ…÷™““Ћб““х•µƒќпјн–‘÷ ”–£Ї
ќё…Ђ”Ќ„і“Їће°Ґ £ї
b£Ѓ ‘є№ ҐЏ ÷–±•ЇЌ Na2CO3µƒ„ч”√ «»№љв““іЉ°ҐљµµЌ““Ћб““х•µƒ»№љвґ»їє”– µƒ„ч”√
c£Ѓ і” ‘є№ҐЏ÷–Ј÷јл≥ц““Ћб““х•µƒ µ—й≤ў„ч «
d£Ѓ …ъ≥…““Ћб““х•µƒїѓ—ІЈі”¶Јљ≥ћ љ
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї2014љмљ≠ќч…ѕ»ƒ÷–—ІЄя“їЅгµг°Ґ µ—й∞аѕ¬∆Џƒ©їѓ—І ‘Њн£®љвќц∞ж£© ћв–Ќ£Ї µ—йћв
£®14Ј÷£©ѕ¬√ж «Љ„°Ґ““°Ґ±ы»эќїЌђ—І÷∆»°““Ћб““х•µƒєэ≥ћ£ђ«лƒг≤ќ”л≤Ґ–≠÷ъЋы√«Ќк≥…ѕаєЎ µ—й»ќќс°£
[ µ—йƒњµƒ]÷∆»°““Ћб““х•
[ µ—й‘≠јн]Љ„°Ґ““°Ґ±ы»эќїЌђ—ІЊщ≤…»°““іЉ(CH3CH218OH)°Ґ““Ћб”л≈®ЅтЋбїмЇѕє≤»»µƒЈљЈ®÷∆»°““Ћб““х•£ђ∆д÷–≈®ЅтЋб≥эЅЋ„чќьЋЃЉЅµƒ„ч”√Ќвїє”–
[„∞÷√…иЉ∆]Љ„°Ґ““°Ґ±ы»эќїЌђ—ІЈ÷±р…иЉ∆ЅЋѕ¬Ѕ–»эћ„ µ—й„∞÷√£Ї
«лі”Љ„°Ґ““ЅљќїЌђ—І…иЉ∆µƒ„∞÷√÷–—°‘с“ї÷÷„чќ™ µ—й “÷∆»°““Ћб““х•µƒ„∞÷√£ђќ“—°‘сµƒ„∞÷√ « (—°ћо °∞Љ„°±їт °∞““°±)°£±ыЌђ—ІљЂЉ„„∞÷√÷–µƒ≤£ЅІє№Єƒ≥…ЅЋ«т–ќЄ…‘пє№≥э∆рјдƒэ„ч”√Ќв£ђЋьµƒЅн“ї÷Ў“™„ч”√ «

[ µ—й≤љ÷и](1)∞і—°‘сµƒ„∞÷√„й„∞“«∆ч£ђ‘Џ ‘є№÷–ѕ»Љ”»л3mLCH3CH218OH£ђ2mL±щі„Ћб£ђ≤Ґ‘Џ“°ґѓѕ¬їЇїЇЉ”»л2mL≈®ЅтЋб≥дЈ÷“°‘»£ђ (2)љЂ ‘є№єћґ®‘ЏћъЉ№…ѕ£ї(3)‘Џ ‘є№ҐЏ÷–Љ”»Ћ Ѕњµƒ±•ЇЌNa2CO3 »№“Ї£ї(4)”√Њ∆ЊЂµ∆ґ‘ ‘є№ҐўЉ”»»£ї(5)µ±єџ≤мµљ ‘є№ҐЏ÷–”–√чѕ‘ѕ÷ѕу ±Ќ£÷є µ—й°£
[ќ ћвћ÷¬џ]a£ЃЄщЊЁ ‘є№ҐЏ÷–єџ≤мµљµƒѕ÷ѕу£ђњ…÷™““Ћб““х•µƒќпјн–‘÷ ”–£Ї
ќё…Ђ”Ќ„і“Їће°Ґ £ї
b£Ѓ ‘ є№ ҐЏ ÷–±• ЇЌ Na2CO3 µƒ„ч”√ «»№љв““іЉ°ҐљµµЌ““Ћб““х•µƒ»№љвґ»їє”– µƒ„ч”√
c£Ѓ і” ‘є№ҐЏ÷–Ј÷јл≥ц““Ћб““х•µƒ µ—й≤ў„ч «
d£Ѓ …ъ≥…““Ћб““х•µƒїѓ—ІЈі”¶Јљ≥ћ љ
≤йњіір∞ЄЇЌљвќц>>
∞ўґ»÷¬–≈ - ЅЈѕ∞≤бЅ–±н - ‘ћвЅ–±н
Їю±± °ї•Ѕ™Ќшќ•Ј®ЇЌ≤їЅЉ–≈ѕҐЊў±®∆љћ® | Ќш…ѕ”–Ї¶–≈ѕҐЊў±®„®«ш | µз–≈’©∆≠Њў±®„®«ш | …жјъ Ј–йќё÷ч“е”–Ї¶–≈ѕҐЊў±®„®«ш | …ж∆у«÷»®Њў±®„®«ш
ќ•Ј®ЇЌ≤їЅЉ–≈ѕҐЊў±®µзї∞£Ї027-86699610 Њў±®” ѕд£Ї58377363@163.com