


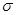 ¼ü»¹ÓŠ1¶Ō¹Ā¶Ōµē×Ó£¬NŌÓ»ÆĄąŠĶĪŖsp3”££Ø4£©CuSO4”¤5H2O×é³ÉĪŖ[Cu(H2O)4]SO4”¤H2O£¬[Cu(H2O)4]2+ÓėSO42-ŅŌĄė×Ó¼ü½įŗĻ£¬Cu2+ÓėH2OŅŌÅäĪ»¼ü½įŗĻ£¬HŗĶOŅŌ¼«ŠŌ¹²¼Ū¼ü½įŗĻ£¬½į¾§Ė®ÖŠµÄHŗĶO·Ö±šŗĶOŗĶHŠĪ³ÉĒā¼ü”££Ø5£©CN-ÖŠCŗĶNŠĪ³ÉČż¼ü£¬Čż¼üÖŠÓŠ1øö
¼ü»¹ÓŠ1¶Ō¹Ā¶Ōµē×Ó£¬NŌÓ»ÆĄąŠĶĪŖsp3”££Ø4£©CuSO4”¤5H2O×é³ÉĪŖ[Cu(H2O)4]SO4”¤H2O£¬[Cu(H2O)4]2+ÓėSO42-ŅŌĄė×Ó¼ü½įŗĻ£¬Cu2+ÓėH2OŅŌÅäĪ»¼ü½įŗĻ£¬HŗĶOŅŌ¼«ŠŌ¹²¼Ū¼ü½įŗĻ£¬½į¾§Ė®ÖŠµÄHŗĶO·Ö±šŗĶOŗĶHŠĪ³ÉĒā¼ü”££Ø5£©CN-ÖŠCŗĶNŠĪ³ÉČż¼ü£¬Čż¼üÖŠÓŠ1øö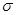 ¼üŗĶ2øö
¼üŗĶ2øö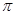 ¼ü”££Ø6£©¾§°ūÖŠCuøöŹżĪŖ1/6”Į12+1/2”Į2+3=6”¢HøöŹżĪŖ1/3”Į6+4=6”£
¼ü”££Ø6£©¾§°ūÖŠCuøöŹżĪŖ1/6”Į12+1/2”Į2+3=6”¢HøöŹżĪŖ1/3”Į6+4=6”£ ¼ü”¢
¼ü”¢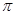 ¼ü ¾§°ū¼ĘĖć
¼ü ¾§°ū¼ĘĖć

 ¾ŁŅ»·“ČżĶ¬²½Ēɽ²¾«Į·ĻµĮŠ“š°ø
¾ŁŅ»·“ČżĶ¬²½Ēɽ²¾«Į·ĻµĮŠ“š°ø æŚĖćÓėÓ¦ÓĆĢāæØĻµĮŠ“š°ø
æŚĖćÓėÓ¦ÓĆĢāæØĻµĮŠ“š°ø ĆūŹ¦µć¾¦×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
ĆūŹ¦µć¾¦×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| ŌŖĖŲ | Ļą¹ŲŠÅĻ¢ |
| X | XŌŖĖŲŠĪ³ÉµÄŅ»ÖÖĶ¬ĖŲŅģŠĪĢåŹĒĢģČ»¾§ĢåÖŠÓ²¶Č×ī“óµÄµ„ÖŹ |
| Y | ³£ĪĀ³£Ń¹ĻĀ£¬Yµ„ÖŹŹĒµ»ĘÉ«¹ĢĢ壬ÓĆÓŚÖĘŌģŗŚ»šŅ© |
| Z | ZµÄ»łĢ¬Ō×ÓŗĖĶāÓŠ3øöÄܼ¶ÉĻÓŠµē×Ó£¬ĒŅÓŠ3øöµ„µē×Ó |
| W | WŌŖĖŲŠĪ³ÉµÄĖ«Ō×Ó·Ö×Ó£¬³£ĪĀĻĀĪŖ»ĘĀĢÉ«ĘųĢ壬Ņ»ÖÖ³£¼ū¹¤ŅµŌĮĻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
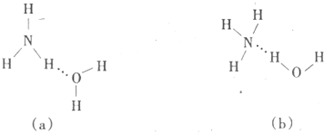
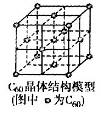
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĶʶĻĢā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
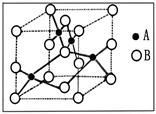
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 )ӣ
)”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com