
 CH3COO-��aq��+H+��aq����H=+0.3 kJ/mol��
CH3COO-��aq��+H+��aq����H=+0.3 kJ/mol������ ��1������Һ�ͼ���Һ������ˮ�ĵ��룬����Һ�е������ӡ�����Һ�е�����������Ũ��Խ��ˮ�ĵ���̶�ԽС���ܹ�ˮ�������Һ�ٽ���ˮ�ĵ��룬ˮ��̶�Խ��ˮ�ĵ���̶�Խ��
��2��c��H+����ͬ������������кͼ��������ͬ��������ʹ��ᣬ��Ϊ������������ʣ����Դ����кͼ������ǿ��
������������ʼ�ˮϡ�ʹٽ����룬����ϡ�ͺ����������Ũ�������������������ӵ�Ũ��ֻ��С��
��3����֪�٣�H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol��
ʵ����ϡ������ϡNaOH��Һ��Ӧ����1mol H2Oʱ�ų�57kJ���ȣ���ڣ�CH3COOH��aq��+OH-��aq���TCH3COO-��aq��+H2O��l����H=-57kJ/mol��
���ݸ�˹���ɣ���-�ٿɵã�CH3COOH��aq��  CH3COO-��aq��+H+��
CH3COO-��aq��+H+��
��4��25��ʱ��NH3•H2O�ĵ��볣��ΪKb=$\frac{c��N{{H}_{4}}^{+}����c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$=1.7��10-3��0.1mol•L-1NH4Cl��Һ��pH=a��������Һ��c��OH-������ϵ���ƽ�ⳣ�����㣻
��5����ǿ��ϡ��ʱ����������Ũ������
��pH=2������Ũ��Ϊ0.01mol/L��pH=13��NaOH��ҺŨ��Ϊ0.1mol/L���������9��1��Ϻ���Һ�ʼ��ԣ�����ʣ������������Ũ�ȣ��ٽ��ˮ�����ӻ�����������Ũ�ȣ�����pH=-lgc��H+����
��pH��ȵ�������Һ������������Ũ����ȣ�CH3COONa��NaHCO3ˮ���Լ��ԣ�Ũ�ȴ����������Ƶģ��������̼�������ˮ��̶�Խ���ε�Ũ��ԽС��
����ҺpH=7����c��H+��=c��OH-������ϵ���غ��жϣ�
�������ᣨH3PO3���Ƕ�Ԫ���ᣬNa2HPO3�����Σ�HPO3-ˮ����Һ�ʼ��ԣ�
�ס�������Һ����ǿ����ʣ���֪����Һ��pH������ҺpH����������Һ�������ϣ������ҺpH����7�������pH֮��=14�����pH������ϵ�������pH�жϣ�
��һ��������ȫ���룬�������벿�ֵ��룬���������غ��жϣ�
��6������Ksp���������ӡ�ͭ���ӳ���ʱ��Ҫ����������Ũ�ȣ���Ҫ����������Ũ��ԽС����������ת��Ϊ������
����Fe3+�պ���ȫ����ʱ����������Ũ�ȣ��ٽ�ϼ���������������������Ũ�Ȼ������ܶȻ��Ƚ��жϣ�
��� �⣺��1����NH3•H2O����KHSO3����ˮ�ĵ��룬��ˮ�ĵ���̶ȴ�������������ĵ���̶ȣ���ˮ��ˮ�ĵ���̶ȸ�С����K2SO3���ڣ�NH4��2SO3 ����ˮ�⣬�ٽ�ˮ�ĵ��룬��NH4��2SO3����笠����Ӻ��������������ٽ�ˮ�⣬ˮ��̶ȸ��ٽ���ˮ�ĵ��룬ˮ�ĵ���̶��ɴ�С����Ϊ���ڢܢۢ٣�
�ʴ�Ϊ���ڢܢۢ٣�
��2��c��H+����ͬ������������кͼ��������ͬ��������ʹ��ᣬ��Ϊ������������ʣ����Դ����кͼ������ǿ����������NaOH��Һ������ɴ�С������˳��Ϊ�٣���=�ۣ�
������������ʼ�ˮϡ�ʹٽ����룬����ϡ�ͺ����������Ũ�������������������ӵ�Ũ��ֻ��С������c��H+���ɴ�С��˳��Ϊ�٣���=�ۣ�
�ʴ�Ϊ���٣���=�ۣ��٣���=�ۣ�
��3����֪�٣�H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol��
ʵ����ϡ������ϡNaOH��Һ��Ӧ����1mol H2Oʱ�ų�57kJ���ȣ���ڣ�CH3COOH��aq��+OH-��aq���TCH3COO-��aq��+H2O��l����H=-57kJ/mol��
���ݸ�˹���ɣ���-�ٿɵã�CH3COOH��aq��  CH3COO-��aq��+H+��aq����H=+0.3 kJ/mol��
CH3COO-��aq��+H+��aq����H=+0.3 kJ/mol��
�ʴ�Ϊ��CH3COOH��aq��  CH3COO-��aq��+H+��aq����H=+0.3 kJ/mol��
CH3COO-��aq��+H+��aq����H=+0.3 kJ/mol��
��4��25��ʱ��NH3•H2O�ĵ��볣��ΪKb=$\frac{c��N{{H}_{4}}^{+}����c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$=1.7��10-3��0.1mol•L-1NH4Cl��Һ��pH=a������Һ��c��OH-��=$\frac{1{0}^{-14}}{1{0}^{-a}}$mol/L=10-14+amol/L����c��NH4+����c��NH3•H2O��=$\frac{{K}_{b}}{c��O{H}^{-}��}$=$\frac{1.7��1{0}^{-3}}{1{0}^{-14+a}}$=1.7��1011-a��
�ʴ�Ϊ��1.7��1011-a��
��5����ǿ����Һȫ�����룬��ˮϡ�ͺ���Һ��H+����Ũ��һ�������ͣ������Ӻ������������ӵ�Ũ�ȵij˻�Ϊ��ֵ��������������Ũ�����ʢٴ���
��pH=2������Ũ��Ϊ0.01mol/L��pH=13��NaOH��ҺŨ��Ϊ0.1mol/L���������9��1��Ϻ���Һ�ʼ��ԣ�ʣ������������Ũ��Ϊ$\frac{0.1��1-0.01��9}{1+9}$mol/L=0.001mol/L����������Ũ��Ϊ10-11mol/L����pH=-lgc��H+��=11���ʢ���ȷ��
��pH��ȵ�������Һ������������Ũ����ȣ�CH3COONa��NaHCO3ˮ���Լ��ԣ�Ũ�ȴ����������Ƶģ���������Ա�̼���ǿ���������ˮ��̶ȱ�̼�������ˮ��̶�С��CH3COONaŨ�ȱ�̼�����Ƶ�С��Ũ�ȣ�NaOH��NaHCO3��CH3COONa���ʢ���ȷ��
����ҺpH=7����c��H+��=c��OH-�����ɵ���غ㣺c��Na+��+c��H+��+c��NH4+��=c��OH-��+2c��SO42-������c��Na+��+c��NH4+��=2c��SO42-�����ʢܴ���
�������ᣨH3PO3���Ƕ�Ԫ���ᣬNa2HPO3�����Σ�HPO3-ˮ����Һ�ʼ��ԣ��ʢ���ȷ��
�ס�������Һ����ǿ����ʣ���֪����Һ��pH������ҺpH�������������p�ֱ�Ϊ2a��a����Һ�������ϣ������ҺpH����7�������pH֮��=14����a+2a=14�����a=$\frac{14}{3}$����Ϊ���Ϊ�ᣬ�������⣬�ʢ���ȷ��
��ij��Ԫ����ˮ�еĵ��뷽��ʽ��H2B�TH++HB-��HB-?H++B2-��һ��������ȫ���룬�������벿�ֵ��룬���������غ㣺c��Na+��=2c��B2-��+2c��HB-�����ʢ���ȷ��
�ʴ�Ϊ���ڢۢݢޢߣ�
��6��25��ʱKsp[Mg��OH��2]=5.6��10-12��Ksp[Cu��OH��2]=2.2��10-20��Ksp[Fe��OH��3]=4.0��10-38��Ksp[Al��OH��3]=1.1��10-33��
�����ӳ���ʱc��OH-��=$\root{3}{\frac{1.1��1{0}^{-33}}{0.1}}$mol/L=$\root{3}{11}$��10-11mol/L��ͭ���ӳ���ʱc��OH-��=$\sqrt{\frac{2.2��1{0}^{-20}}{0.1}}$mol/L=$\sqrt{22}$��10-10�������ӳ�����Ҫ����������Ũ��С������������Al��OH��3������
Fe3+�պ���ȫ����ʱ����������Ũ��Ϊ$\root{3}{\frac{4��1{0}^{-38}}{1{0}^{-5}}}$mol/L=$\root{3}{4}$��10-11mol/L����c��Al3+����c3��OH-��=0.2��$\frac{4��1{0}^{-38}}{1{0}^{-5}}$=8��10-34��Ksp[Al��OH��3]=1.1��10-33��û����������������
�ʴ�Ϊ��Al��OH��3�������У�
���� ���⿼����ҺpH���㡢����ˮ�⡢������ʵĵ��롢����Ũ�ȴ�С�Ƚϡ��ܶȻ��йؼ���ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 40.625 | B�� | 42.15 | C�� | 38.225 | D�� | 42.625 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 10.00 | 0.50 | 20.40 |
| �ڶ��� | 10.00 | 4.00 | 24.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��aL0.1mol/L��CH3COOH��Һ��bL0.1mol/L��KOH��Һ��ϣ�������Һ��һ�����ڣ�c��K+��+c��H+���Tc��CH3COO-��+c��OH-�� | |
| B�� | ��0.1mol/L��NaHCO3��Һ��0.3mol/L��Ba��OH��2��Һ�������ϣ�������Һ��һ�����ڣ�c��OH-����c��Ba+����c��Na+����c��H+�� | |
| C�� | ��1mol/L��CH3COOH��Һ�м�������CH3COONa���壬����CH3COONaˮ���Լ��ԣ�������Һ��pH���� | |
| D�� | �����£���pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ�ĵ���̶���ͬ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 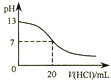 ͼ��ʾ25��ʱ����0.1 mol•L-1����ζ�20 mL 0.1 mol•L-1 NaOH��Һ����Һ��pH�����������ı仯 | |
| B�� | 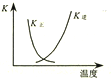 ͼ�����߱�ʾ��Ӧ2SO2��g��+O2��g���T2SO3��g������H��0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯 | |
| C�� | 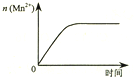 ͼ�۱�ʾ10 mL 0.01 mol•L-1 KMnO4 ������Һ�������0.1 mol•L-1 H2C2O4��Һ���ʱ��n��Mn2+�� ��ʱ��ı仯 | |
| D�� | 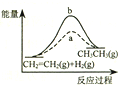 ͼ��a��b���߷ֱ��ʾ��ӦCH2=CH2 ��g��+H2��g����CH3CH3��g������H��0ʹ�ú�δʹ�ô���ʱ����Ӧ�����е������仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ABCDE������Һ�ֱ���NaOH��Һ����ˮ�����ᡢ���ᡢNH4HSO4��Һ��һ�֣������½�������ʵ�飺
ABCDE������Һ�ֱ���NaOH��Һ����ˮ�����ᡢ���ᡢNH4HSO4��Һ��һ�֣������½�������ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
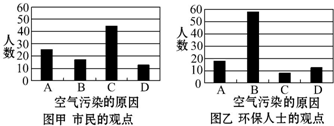
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
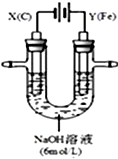 ij����С��ֱ�����ͼ��ʾװ�öԵ��ԭ������ʵ��̽����
ij����С��ֱ�����ͼ��ʾװ�öԵ��ԭ������ʵ��̽�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com