������Һ�о����ڵ�����е�ǰ�أ�������Һ�������ض������Χ������г��ܳ桢��桢���ࡢ�ݱ���ȶ�ֲ����˼��ѵ���Ȼ����ʵ���ң��Һ��ס�����ض����Χ������FeS����ͭ��п��ȿ��
��1���ӵ縺�ԽǶȷ�����C��N��OԪ�صķǽ�����������ǿ������˳��Ϊ
����һ�����ܴ�С��˳��Ϊ
��
��2��ͭԭ�Ӻ�������Ų�ʽΪ��
��
��3��FeS��NaCl��Ϊ���Ӿ��壬�����ṹ���ƣ�ǰ���۵�Ϊ985��C������801��C����ԭ����
��
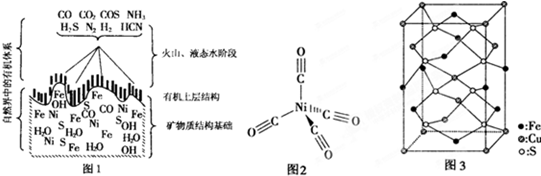
��4���ӡ�����ض���γɵĿ�����ȡ�����轫��ת��Ϊ���ʻ��������ṹ��ͼ2���������������λ��Ϊ
�����ʻ�����������ΪҺ̬��������CCl
4�������л��ܼ�����̬���ʻ���������
���壮
��5����ͭ��������Ҫ��ͭ��ȫ����
��ͭ�����������ģ���ͼ3Ϊ��ͭ��ľ�����
��д����ͭ��Ļ�ѧʽ
����ͭ��ľ���Ϊֱ�������Σ������������ⳤΪa cm����Ϊbcm����ͭ���Ħ������ΪMg/mol�������ӵ�����ΪNAmol
-1�����ͭ������ܶ�
g/cm
3��




 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
 ����˹ƥ�ַ���������NaOH��Һ����У��ܷ�����Ӧ�Ļ�ѧ���ǣ�������
����˹ƥ�ַ���������NaOH��Һ����У��ܷ�����Ӧ�Ļ�ѧ���ǣ�������

