
 3£¬3£¬5£¬5-Ėļ׻łøżĶ飻
3£¬3£¬5£¬5-Ėļ׻łøżĶ飻·ÖĪö £Ø1£©¢Łøł¾ŻĶéĢžµÄĆüĆūŌŌņ·ÖĪö£ŗĆüĆūŅŖ·ūŗĻ”°Ņ»³¤”¢Ņ»½ü”¢Ņ»¶ą”¢Ņ»Š””±£¬Ņ²¾ĶŹĒÖ÷Į“×ī³¤£¬±ąŗÅĘšµćĄėÖ§Į“×ī½ü£¬Ö§Į“ŹżÄæŅŖ¶ą£¬Ö§Į“Ī»ÖĆŗÅĀėÖ®ŗĶ×īŠ”£¬¼ņµ„ŌŚĒ°Ķ¬Ļą²¢£¬Ęä¼äÓ¦»®Ņ»¶ĢĻߣ»
¢Śŗ¬ÓŠ¹ŁÄÜĶŵÄÓŠ»śĪļĆüĆūŹ±£¬ŅŖŃ”ŗ¬¹ŁÄÜĶŵÄ×ī³¤Ģ¼Į“×÷ĪŖÖ÷Į“£¬¹ŁÄÜĶŵÄĪ»“Ī×īŠ”£»
£Ø2£©¢ŁøĆÓŠ»śĪļĪŖĶéĢž£¬øł¾ŻĶéĢžµÄĆüĆūŌŌņ¶ŌøĆÓŠ»śĪļ½ųŠŠ½į¹¹¼ņŹ½µÄŹéŠ“£»
¢ŚĶéĢžÖŠ³öĻÖŅŅ»ł£¬ŌņÖ÷Į“ÖĮÉŁŗ¬ÓŠ5øöC£¬¾Ż“ĖŠ“³öøĆÓŠ»śĪļµÄ½į¹¹¼ņŹ½£»
£Ø3£©¢ŁŅŅ“¼ÓėÅØĮņĖį¼ÓČČ·¢ÉśĻūČ„·“Ó¦ÖĘČ”ŅŅĻ©£»
¢ŚŅŅ“¼“ß»ÆŃõ»Æ£¬Éś³ÉŅŅČ©ŗĶĖ®£»
¢ŪĀ±“śĢžŌŚ¼īµÄ“¼ČÜŅŗÖŠ¼ÓČČ·¢ÉśĻūČ„·“Ӧɜ³ÉĻ©Ģž£»
¢ÜŅŅČ©ŗĶŠĀÖĘĒāŃõ»ÆĶ·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉŅŅĖį”¢Ńõ»ÆŃĒĶŗĶĖ®£®
½ā“š ½ā£ŗ£Ø1£©¢ŁÖ÷Į“×ī³¤µÄĢ¼Į“ŗ¬ÓŠ7øöC£¬ĪŖøżĶ飬ŌŚ3”¢5ŗÅCÉĻø÷ÓŠ2øö¼×»ł£¬ĖłŅŌĆū³ĘĪŖ£ŗ3£¬3£¬5£¬5-Ėļ׻łøżĶ飬
¹Ź“š°øĪŖ£ŗ3£¬3£¬5£¬5-Ėļ׻łøżĶ飻
¢Ś×ī³¤Ģ¼Į“ŗ¬ÓŠ5øöC£¬Ö÷Į“ĪŖĪģĻ©£¬ŌŚ4ŗÅCÉĻÓŠ1øö¼×»ł£¬ŌŚ2£¬3ŗÅCÉĻÓŠĖ«¼ü£¬ĖłŅŌĆū³ĘĪŖ£ŗ4-¼×»ł-2-ĪģĻ©£¬
¹Ź“š°øĪŖ£ŗ4-¼×»ł-2-ĪģĻ©£»
£Ø2£©¢Ł2£¬3-¶ž¼×»ł-4-ŅŅ»łŅŃĶ飬×ī³¤µÄÖ÷Į“ŗ¬ÓŠ6øöCŌ×Ó£¬2”¢3ŗÅĢ¼Ō×ÓÉĻø÷Į¬½Ó1øö¼×»ł£¬ŌŚ4ŗÅCÉĻÓŠ1øöŅŅ»ł£¬Ęä½į¹¹¼ņŹ½ĪŖ£ŗCH3CH£ØCH3£©CH£ØCH3£©CH£ØC2H5£©CH2CH3£¬
¹Ź“š°øĪŖ£ŗCH3CH£ØCH3£©CH£ØCH3£©CH£ØC2H5£©CH2CH3£»
¢ŚĶéĢžÖŠŗ¬ÓŠŅŅ»ł£¬ŌņŅŅ»łÖĮÉŁŌŚ3ŗÅĪ»£¬ĖłŅŌÖ»ÓŠŅ»øöŅŅ»łĒŅŹ½Įæ×īŠ”µÄĶéĢžµÄ½į¹¹¼ņŹ½ĪŖCH3CH2CH£ØC2H5£©CH2CH3£¬
¹Ź“š°øĪŖ£ŗCH3CH2CH£ØC2H5£©CH2CH3£»
£Ø3£©¢ŁŹµŃéŹŅĄūÓĆŅŅ“¼ÄÜ·¢ÉśĻūČ„·“Ó¦ÖĘŅŅĻ©£ŗCH3-CH2-OH $”ś_{170”ę}^{ÅØĮņĖį}$CH2-CH2”ü+H2O£¬
¹Ź“š°øĪŖ£ŗCH3-CH2-OH $”ś_{170”ę}^{ÅØĮņĖį}$CH2-CH2”ü+H2O£»
¢ŚŅŅ“¼ÖŠŗ¬-OH£¬ÄÜ·¢Éś“ß»ÆŃõ»ÆÉś³ÉŅŅČ©£¬Ęä·“Ó¦·½³ĢŹ½ĪŖ£ŗ2CH3CH2OH+O2$”ś_{”÷}^{Cu»ņAg}$2CH3CHO+2H2O£¬
¹Ź“š°øĪŖ£ŗ2CH3CH2OH+O2$”ś_{”÷}^{Cu»ņAg}$2CH3CHO+2H2O£»
¢Ū2-ĀȱūĶéÓėNaOHµÄŅŅ“¼ČÜŅŗ¹²ČČ·¢ÉśĻūČ„·“Ó¦£¬·½³ĢŹ½ĪŖ£ŗCH3-CHCl-CH3+NaOH$”ś_{”÷}^{ŅŅ“¼}$CH3-CH=CH2”ü+NaCl+H2O£¬
¹Ź“š°øĪŖ£ŗCH3-CHCl-CH3+NaOH$”ś_{”÷}^{ŅŅ“¼}$CH3-CH=CH2”ü+NaCl+H2O£»
¢Ü£©ŌŚ¼ÓČČĢõ¼žĻĀ£¬ŅŅČ©ŗĶŠĀÖĘĒāŃõ»ÆĶ·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉŅŅĖį”¢Ńõ»ÆŃĒĶŗĶĖ®£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗCH3CHO+2Cu£ØOH£©2$\stackrel{”÷}{”ś}$CH3COOH+Cu2O+2H2O£¬
¹Ź“š°øĪŖ£ŗCH3CHO+2Cu£ØOH£©2$\stackrel{”÷}{”ś}$CH3COOH+Cu2O+2H2O£®
µćĘĄ ±¾Ģāæ¼²éĮĖÓŠ»śĪļĆüĆū”¢½į¹¹¼ņŹ½µÄŹéŠ“”¢»Æѧ·“Ó¦·½³ĢŹ½µÄŹéŠ“£¬øĆĢā×¢ÖŲĮĖ»ł“”ŠŌŹŌĢāµÄ漲飬²ąÖŲ¶Ōѧɜ»ł“”ÖŖŹ¶µÄ¼ģŃéŗĶѵĮ·£¬øĆĢāµÄ¹Ų¼üŹĒĆ÷Č·ÓŠ»śĪļµÄĆüĆūŌŌņ£¬Č»ŗó½įŗĻÓŠ»śĪļµÄ½į¹¹¼ņŹ½Įé»īŌĖÓĆ£¬ÓŠĄūÓŚÅąŃųѧɜµÄ¹ę·¶“šĢāÄÜĮ¦£¬×¢ŅāÓŠ»śĪļ·“Ó¦ĄąŠĶ¼°·“Ó¦Ģõ¼ž£¬ĢāÄæÄŃ¶Č²»“ó£®


 A¼Ó½šĢā ĻµĮŠ“š°ø
A¼Ó½šĢā ĻµĮŠ“š°ø Č«ÓŲāŹŌ¾ķĻµĮŠ“š°ø
Č«ÓŲāŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £¬FĖ®ČÜŅŗĻŌŠŌ¼īŠŌ£ØĢī£ŗĖįŠŌ”¢ÖŠŠŌ»ņ¼īŠŌ£©£®
£¬FĖ®ČÜŅŗĻŌŠŌ¼īŠŌ£ØĢī£ŗĖįŠŌ”¢ÖŠŠŌ»ņ¼īŠŌ£©£®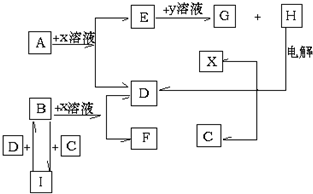
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 ¢Ś
¢Ś ¢Ū
¢Ū ¢Ü
¢Ü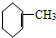 ¢Ż
¢Ż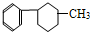 ¢Ž
¢Ž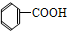 ¢ß
¢ß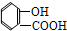 ¢ą
¢ą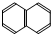 ¢į
¢į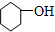 ¢ā
¢ā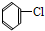
| A£® | ¢Ś¢Ū¢Ü¢Ż¢ą¢ā | B£® | ¢Ś¢Ż¢ą | C£® | ¢Ū¢Ü¢ą¢ā | D£® | ¢Ś¢ą¢ā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
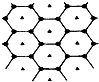 Ģ¼µÄŅ»ÖÖµ„ÖŹŹÆÄ«³Ź²ćד½į¹¹£¬ÓŠŅ»Ģ¼Ć¾ŠĀŠĶ²ÄĮĻ¾ĶŹĒŌŚŹÆÄ«Ģ¼Ō×Ó²ć¼ä¼ÓČėĆ¾Ō×Ó²ć£¬Į½²ćø©ŹÓĶ¼£ŗøĆ²ÄĮĻµÄ»ÆѧŹ½ĪŖMgC2£®
Ģ¼µÄŅ»ÖÖµ„ÖŹŹÆÄ«³Ź²ćד½į¹¹£¬ÓŠŅ»Ģ¼Ć¾ŠĀŠĶ²ÄĮĻ¾ĶŹĒŌŚŹÆÄ«Ģ¼Ō×Ó²ć¼ä¼ÓČėĆ¾Ō×Ó²ć£¬Į½²ćø©ŹÓĶ¼£ŗøĆ²ÄĮĻµÄ»ÆѧŹ½ĪŖMgC2£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ū | B£® | ¢Ś¢Ū¢Ž | C£® | ¢Ś¢Ü¢Ż | D£® | ¢Ū¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
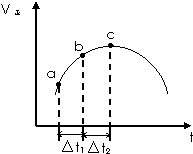 Ļņ¾ųČČŗćČŻĆܱÕČŻĘ÷ÖŠĶØČėSO2ŗĶNO2£¬ŌŚŅ»¶ØĢõ¼žĻĀŹ¹·“Ó¦SO2£Øg£©+NO2£Øg£©?SO3£Øg£©+NO£Øg£©“ļµ½Ę½ŗā£¬Õż·“Ó¦ĖŁĀŹĖꏱ¼ä±ä»ÆµÄŹ¾ŅāĶ¼ČēĶ¼ĖłŹ¾£®
Ļņ¾ųČČŗćČŻĆܱÕČŻĘ÷ÖŠĶØČėSO2ŗĶNO2£¬ŌŚŅ»¶ØĢõ¼žĻĀŹ¹·“Ó¦SO2£Øg£©+NO2£Øg£©?SO3£Øg£©+NO£Øg£©“ļµ½Ę½ŗā£¬Õż·“Ó¦ĖŁĀŹĖꏱ¼ä±ä»ÆµÄŹ¾ŅāĶ¼ČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£® £®
£®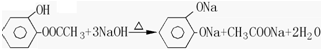 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com