
ʵ���ҿ�����MgCl2?6H2OΪԭ���Ʊ�̼��þ���룬��Ҫ����������
�Իش�
(1)����1��������_______��
(2)������Ӧ����Ҫ���ƺ��¶ȣ���ΪMgCO3����ˮ�г�ʱ�������п���ȫ����Mg(OH)2����ԭ����_____________________��
(3)��������ף��ڼ������ʱҲ�������ɼ�ʽ̼��þMg5(OH)2(CO3)4��4H2O��д���÷�Ӧ�Ļ�ѧ����ʽ______________��
(4)ij������Ϊ��ȷ��MgCO3��ˮ�г�ʱ�������к����õĹ���ɷ֣����������о�����ע��Mg(OH)2, Mg5(OH)2(CO3)4.4H2O�����ʱ������ֽ⣩
�ٶ����о�����������±������ݡ�
| ʵ�鲽�� | Ԥ�ڵ�ʵ������ͽ��� |
| ȡһ�������Ĺ�����Ʒ | �� ����������ΪMg(OH)2 |
��14�֣�
��1�����ˣ�2�֣�
��2��MgCO3�ڼ�����й������ܷ���ˮ�ⷴӦ������ˮ�����ɵ�Mg(OH)2�ܽ�ȸ�С��������CO2�������ɣ�ʹˮ��������ȫ�� MgCO3ת��ΪMg(OH)2�� ��3�֣�
��3�� 5 MgCO3 + 5H2O=Mg5(OH)2(CO3)4��4H2O + CO2�� ��2�֣�
��4���� ��4�֣�ʵ�鲽�� Ԥ�ڵ�ʵ������ͽ��� �����Թ��У��μ�������ϡ����
���������Թ��г�ּ��ȣ�������������ͨ�뵽ʢ����������ʯ��ˮ���ձ��� ����������
�������ʯ��ˮ������� ��
�����������𰸾����֣�
�� �ܣ�1�֣� ��������������þ���ʽ̼��þ����������ʱ�����������ļ�������ȷ���ģ��Ҳ���ͬ����2�֣�
��������������ڽ��̽�����ʱ��Ҫ�ܹ��������л�ȡ���֪ʶ��ϵ�α�֪ʶ���н��⡣���⽫Mg2+��CO32-�������MgCO3��������̼���ˮ��ʼ��ԺͿα���ˮ�������ɵ����֪ʶ����֪��ת��ΪMg(OH)2�Լ���Ŀ���ᵽ��Mg5(OH)2(CO3)4��4H2O��������������ʲô���������̽����̽���Ľ�������������̼����ļ��飬�Ӷ����⡣
���㣺������̽��Ϊ����������Ԫ�ؼ�������֪ʶ�����ʵļ����Լ�̽���Ļ������̡�


 ȫ��������ϵ�д�
ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС����̽���ں�Mg2+��Al3+ �Ļ����Һ�еμ�NaOH��Һʱ������������������Ĺ��̡�
��ʵ�顿��0.1 mol?L��1 MgSO4��0.05 mol?L��1Al2(SO4)3�Ļ����Һ�еμ�0.5 mol?L-1NaOH��Һ�����������Ӵ���������Ӧ���������������Һ��pH��NaOH��Һ�ļ���仯�����ͼ��ʾ��
��1��Ϊ��ȷ���Ƽ���NaOH��Һ��������ɽ�NaOH��Һ���� �����������ƣ��еμӡ�
��2��ͼ��������pH���ӻ����ĽΣ���һ�Σ�a��ǰ����Ӧ��ʵ�������� ��
��3���Եڶ��Σ�b��c֮�䣩��ҺpH�仯�����ı��ʣ�С��ͬѧ���������Ʋ⣬�벹���Ʋ�2��3��
�Ʋ�1������Mg(OH)2����������OH����
�Ʋ�2�� ��
�Ʋ�3�� ��
���Ʋ�1����ʵ���������a��֮ǰ��Ӧ�����ӷ���ʽΪ ����ݴ�����Mg(OH)2��Al(OH)3����������ˮ��Һ���ܽ��ԵIJ��� ��
��4�������e�����Һ�нϴ������ڵĺ�����Ԫ�ص����Ӳ����ʵ����飨�ɲ���������
| �ϴ������ڵĺ�����Ԫ�ص����� | ���鷽�� |
| | |
| | |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������һ���ս̰桶��ѧ1�����ޣ�����ҵ�ϴӺ���Ʒ���������л�ȡ�������ͼ���£�
��1�������������̵ĵ�һ���ǡ����ݡ�������ʵ��Ŀ���� ��
��2������������һ�����пɹ�ѡ�õ��Լ���Cl2��Br2��Ũ�����H2O2���μ�ϡ���ᣩ��������Ⱦ�Ƕȿ��ǣ�����ѡ��ĺ����Լ� ��
��3�����������������У����ᴿ��õ��ʵ⡱һ����Ϊ��������������ΪӦѡ����ͼʵ��װ�÷ֱ��ǣ� ���� �� �����������Ⱥ�˳����д����


 �����϶����ҹ�ʵ�ʹ�ҵ���ú�����ȡ�⣬���õ������ӽ����������������£�
�����϶����ҹ�ʵ�ʹ�ҵ���ú�����ȡ�⣬���õ������ӽ����������������£�
��4��ʵ�ʹ�ҵ�����У��ữ�������ķ������ȼ��������ữ��ʹpH���͵�2��Ȼ������������һ�����������ʹ��������������ҵͨ��������������ԭ���ǣ������ӷ���ʽ��ʾ��
��
��5���������������������ӽ�����֬���ü�����֬������������������һ�ԭ��������������֬�����ĵ�Ԫ��״̬�� ����д������̬������̬������������Ӧ���� ����д���б�ţ�A����������B����ԭ��������ʵ�ֵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ȼ�ͭ��һ�ֹ㷺�����������ϡ�ľ�ķ������ȵĻ�����Ʒ��ij�о�С���ô�ͭ(������Fe)�����������Ʊ��Ȼ�ͭ����(CuCl2 ? 2H2O)��
��1)Ϊ����ɲ���I,�ɲ�������ͼ��ʾ��װ�ý���ͭת��Ϊ����a��
�ڸ�ʵ���У�����A��Cװ���еľƾ���ʱ��Ӧ���ȵ�ȼ_______װ�õľƾ���,Dװ�õ�������
_______,Cװ�������ɵIJ�����_______��
(2)����II�еIJ�����:������I�еĹ�����뵽�����У�Ȼ�����_______��������ȫ�ܽ⡣
(3)����III�м�����Լ���H2O2,��Ŀ������������Ʊ����Ȼ�ͭ����Ĵ��ȣ���д���йط�Ӧ�����ӷ���ʽ______________��
(4) ����IV��Ӧ�ȼ���ij�Լ�,Ȼ����˵õ���Һ��Ϊ�˵�����Һ��pHֻʹFe3+��ȫ������������Լ���CuO��ĩ�����ð�ˮ��NaOH��Һ����������_______��
(5)����V��,����Һ����Ũ������ȴ�ᾧ�����˵õ�CuCl2��2H2O���塣�����õ���ˮCuCl2����ʵ����Ҳ���Բ�������װ�����CuCl2 ? 2H2O��ˮ�õ���ˮCuCl2����Aװ���е����ֻ�ѧ�Լ���_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ�����Ʊ������飨C2H5Br����װ�úͲ�����ͼ������֪������ķе�38.4�棩
�ټ��װ�õ������ԣ���װ��ͼ��ʾ��U�ܺʹ��ձ��м����ˮ��
����Բ����ƿ�м���10mL95%�Ҵ���28mLŨ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ��
��С����ȣ�ʹ���ַ�Ӧ��
�ش��������⣺
��1����ʵ����ȡ������Ļ�ѧ����ʽΪ�����ɵ���ΪNaHSO4����______________________��
��2����Ӧʱ���¶ȹ��ߣ��ɿ����к���ɫ��������������廯ѧʽΪ__________��
��3��Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��__________��
��4��U���ڿɹ۲쵽��������_____________________________��
��5����Ӧ������U�ι��д��Ƶ�C2H5Br���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ���ѡ�������Լ��е�_________________������ţ�
a���� b��H2O c��Na2SO3��Һ d��CCl4
�������Ҫ����������______________�����������ƣ���
��6�����м���ʵ�鲽�裬�����ڼ�������������Ԫ�أ�����ȷ�IJ���˳���ǣ�ȡ���������飬Ȼ��__________________������ţ���
�ټ��ȣ��ڼ���AgNO3��Һ���ۼ���ϡHNO3�ữ���ܼ���NaOH��Һ������ȴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��(Ti)����Ϊ21���ͽ�����ұ���ѵ���Ҫԭ���Ǻ�Fe2O3��������FeTiO3)���������������£�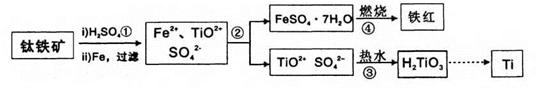
��֪��TiOSO4������ˮ����ˮ�⣬H2TiO3������ˮ���Իش��������⣺
(1)����ڵ���Ҫʵ���������ȴ���ᾧ�� (���������)���������ʵ�����г����Լ����� (���������ƣ��м�ǿ�ȡ�
(2)������м�����ˮ�������� ��
(3)�������������������(FeSO4��7H2O)�ڿ����������������졢ˮ����������д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(4)��������õ���������������(FeSO4��7H2O)��Ŀǰ��ҵ�ϴ��������ж���ˮ���õĻ�ѧ�Լ������߷�Ӧ��(Cr��+6��ת��Ϊ+3��)����ת��Ϊ����Ҫ��ҵ��ֵ�������帴�����������FeO��FeyCrxO3��ʾ)�����Ʊ��������帴����������������Եĺ�����ˮ�У�����FeSO4��7H2O������ӦΪ��ˮ�����۸����൱��CrO3)������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ����ۣ���ȷ����̣�����������
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� | �ж� |
| �� | ij�����������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ˵���ü����� | |
| �� | ��ij��Һ�м������ᣬ�����������������м���BaCl2��Һ�а�ɫ���������� | ֤������Һ���� SO42�� | |
| �� | �������Һ�м���һ������ϡ������ȣ��ټ���һ����������������ͭ���ȡ� | ֤������ˮ����������� | |
| �� |  ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������ ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������ ��Һ ��Һ | �����Ƶ������Ƿ�Ϊ��ϩ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ�ӱ������˽�����и��������Բ����κ�̼���Σ�������ʵ����֤��һ��ʵ���������κ�������һ���������ϵ�֪�����ᣨH2C2O4��������ǿ�ڴ���Ķ�Ԫ�л��ᣬ����һ�ֻ�ԭ�Խ�ǿ�����ʣ���2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O������ƣ�CaC2O4��������ˮ�ʹ��ᣬ������ǿ�CaC2O4+2H+= H2C2O4+Ca2+��
��1�����ʵ�鷽����֤�����к��в����κ�̼���Σ��������ʵ�鲽�衢Ԥ������ͽ��ۡ�
��ѡ�Լ���1 mol��L��1 H2SO4��1 mol��L��1 HCl��0.1 mol��L��1 NaOH��1 mol��L��1 CaCl2��0.01 mol��L��1 KMnO4������ʯ��ˮ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1����������ĥ���ݡ����˵õ����������Һ�� | |
| ����2��������Һ�������ԣ��μ�����CaCl2��Һ�� | ���ְ�ɫ������˵�������п��ܺ��в����κ�̼���Ρ� |
| ����3��ȡ����2�ij������Թ��У� | |
| ����4�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ�����ڸ���ռ������ն��������װ�ã����з�����ȷ����
| ѡ�� | X | �ռ����� | Y |
| A | ��ʯ�� | �Ȼ��� | ˮ |
| B | ��ʯ�� | ���� | ˮ |
| C | �Ȼ��� | �������� | �������� |
| D | �Ȼ��� | һ������ | �������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com