
| A���٢� | B���ڢܢ� |
| C���ڢۢܢ� | D���٢ڢۢܢ� |
| c(��)��V(��) |
| V(��) |
| c(��)��V(��) |
| V(��) |
| c(��)��V(��) |
| V(��) |
| c(��)��V(��) |
| V(��) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
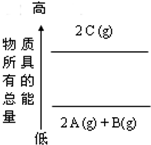
| A��2A��g��+B��g���T2C��g����H=a��a��0�� |
| B��2C��g���T2A��g��+B��g����H=a��a��0�� |
| C��2A+B�T2C��H=a��a��0�� |
| D��2C�T2A+B��H=a��a��0�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��TNT 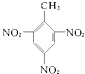 |
B��Ӳ֬�������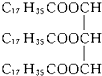 |
| C��������� CH3OOCCH3 |
| D��3-����ȩ ��CH3��2CHCH2COH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
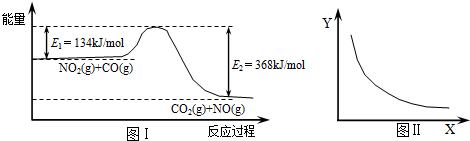
| A���÷�Ӧ���ʱ��H=+234 kJ?mol-1 |
| B����X��ʾ��ϵ��ѹǿ����Y��ʾ�Ŀ�����NO2��ת���� |
| C����X��ʾ�¶���Y��ʾ�Ŀ�����CO2���ʵ���Ũ�� |
| D������CO����ʼŨ�ȣ�ƽ��������Ӧ�����ƶ�����Ӧ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
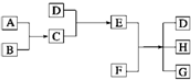 ��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��
��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��������������������Ȣ��ж� |
| B���١��ܻ�Ϊͬϵ�� |
| C���١��ڡ��ۡ��ܾ���������������Һ��Ӧ |
| D�������ʵ����ڡ��۷ֱ���������������ȫȼ�գ�ǰ�����������Ⱥ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com